2023-11-08 ブラウン大学
◆この新しい理解は、従来の電池内部の材料を置き換えることで、長寿命でエネルギー密度の高い電池を実現するための欠けていた部分を埋めることができるかもしれない。
<関連情報>
- https://www.brown.edu/news/2023-11-08/micelle-structures
- https://www.nature.com/articles/s41563-023-01700-3
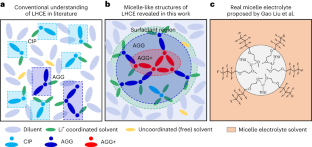
局在化した高濃度電解質がミセル様構造によってさらに局在化する。 Localized high-concentration electrolytes get more localized through micelle-like structures
Corey M. Efaw,Qisheng Wu,Ningshengjie Gao,Yugang Zhang,Haoyu Zhu,Kevin Gering,Michael F. Hurley,Hui Xiong,Enyuan Hu,Xia Cao,Wu Xu,Ji-Guang Zhang,Eric J. Dufek,Jie Xiao,Xiao-Qing Yang,Jun Liu,Yue Qi & Bin Li
Nature Materials Published06 November 2023
DOIhttps://doi.org/10.1038/s41563-023-01700-3
Abstract
Liquid electrolytes in batteries are typically treated as macroscopically homogeneous ionic transport media despite having a complex chemical composition and atomistic solvation structures, leaving a knowledge gap of the microstructural characteristics. Here, we reveal a unique micelle-like structure in a localized high-concentration electrolyte, in which the solvent acts as a surfactant between an insoluble salt in a diluent. The miscibility of the solvent with the diluent and simultaneous solubility of the salt results in a micelle-like structure with a smeared interface and an increased salt concentration at the centre of the salt–solvent clusters that extends the salt solubility. These intermingling miscibility effects have temperature dependencies, wherein a typical localized high-concentration electrolyte peaks in localized cluster salt concentration near room temperature and is used to form a stable solid–electrolyte interphase on a Li metal anode. These findings serve as a guide to predicting a stable ternary phase diagram and connecting the electrolyte microstructure with electrolyte formulation and formation protocols of solid–electrolyte interphases for enhanced battery cyclability.