2026-01-14 横浜国立大学
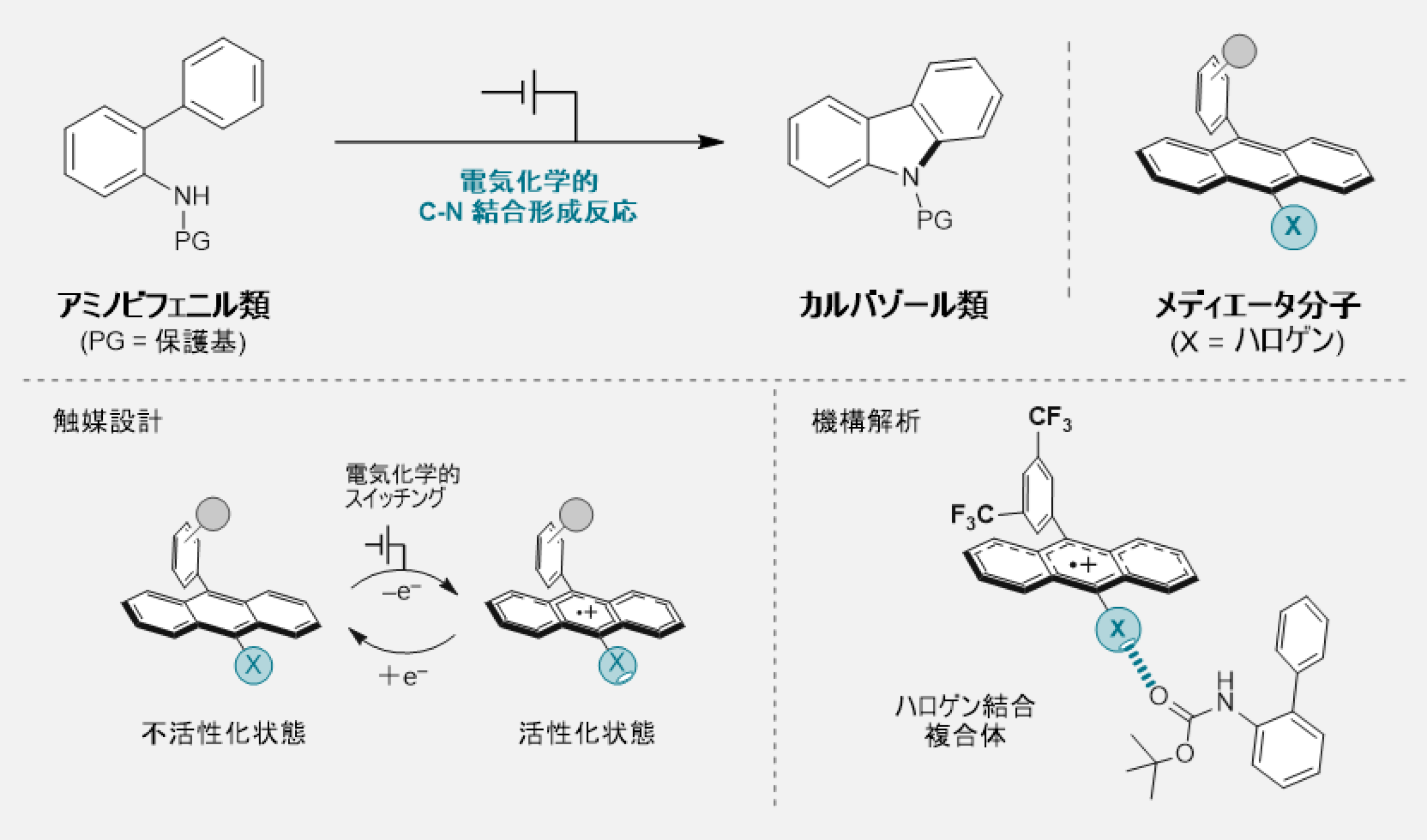
図1. 今回開発した触媒とそれを用いたC-N結合形成反応
<関連情報>
- https://www.ynu.ac.jp/hus/koho/34567/detail.html
- https://www.ynu.ac.jp/hus/koho/34567/34_34567_1_1_260109012840.pdf
- https://pubs.acs.org/doi/10.1021/jacs.5c18175
ハロアントラセンメディエーターにおける酸化還元スイッチング可能なハロゲン結合が効率的な電気触媒C-Nカップリングを可能にする Redox-Switchable Halogen Bonding in Haloanthracene Mediators Enables Efficient Electrocatalytic C–N Coupling
Atsuki Hirama,Kayo Suda,Shohei Yoshinaga,Moto Kikuchi,Su-Gi Chong,Azusa Kikuchi,Yusuke Ishigaki,Daisuke Yokogawa,Mahito Atobe,and Naoki Shida
Journal of the American Chemical Society Published: January 8, 2026
DOI:https://doi.org/10.1021/jacs.5c18175
Abstract
We report the development of redox mediators based on 9-halo-10-arylanthracenes that engage in halogen bonding only upon one-electron oxidation. This redox-switchable interaction enables an effective substrate preorganization and promotes intramolecular C–N bond formation via electrocatalysis. Systematic evaluation of halogenated mediators (1a–1c) across various N-protected 2-aminobiphenyl substrates revealed that the iodoanthracene derivative 1a exhibited superior catalytic performance. Building on this, we synthesized a series of 10-aryl-substituted iodoanthracenes (1d–1h) to further optimize the mediator structure. Kinetic analysis by foot-of-the-wave analysis identified 1h, bearing a 3,5-bis(trifluoromethyl)phenyl group, as a highly active mediator with an apparent rate constant over an order of magnitude higher than that of its counterparts. Bulk electrolysis experiments confirmed its remarkable performance, achieving high yields in short reaction times. Computational studies demonstrated that halogen bonding is markedly strengthened in the radical cation state, and that this interaction significantly enhances the acidity of the N–H bond in the substrates, enabling proton-coupled electron transfer (PCET) even with a weak base. Energy diagrams constructed from density functional theory calculations supported a mechanism in which the mediator not only facilitates PCET but also stabilizes cationic intermediates throughout the catalytic cycle. This work establishes a new design paradigm for redox mediators, where redox-induced noncovalent interactions can be harnessed to control both reactivity and selectivity. The concept of halogen-bonding-assisted PCET provides a powerful platform for advancing molecular electrocatalysis.