2025-12-23 名古屋大学
<関連情報>
- https://www.nagoya-u.ac.jp/researchinfo/result/2025/12/post-924.html
- https://www.nagoya-u.ac.jp/researchinfo/result/upload_images/20251223_itbm.pdf
- https://onlinelibrary.wiley.com/doi/10.1002/anie.202522746
キラルホウ素含有多環芳香族炭化水素からの二重円偏光発光 Dual Circularly Polarized Luminescence from Chiral Boron-Embedded Polycyclic Aromatic Hydrocarbons
Dr. Tatsuya Mori, Yoshiharu Sano, Prof. Dr. Tomoyuki Ikai, Dr. Yuuya Kawasaki, Prof. Dr. Katsuhiko Tomooka, Prof. Dr. Takahiro Sasamori, Prof. Dr. Shigehiro Yamaguchi
Angewandte Chemie International Edition Published: 21 December 2025
DOI:https://doi.org/10.1002/anie.202522746
Abstract
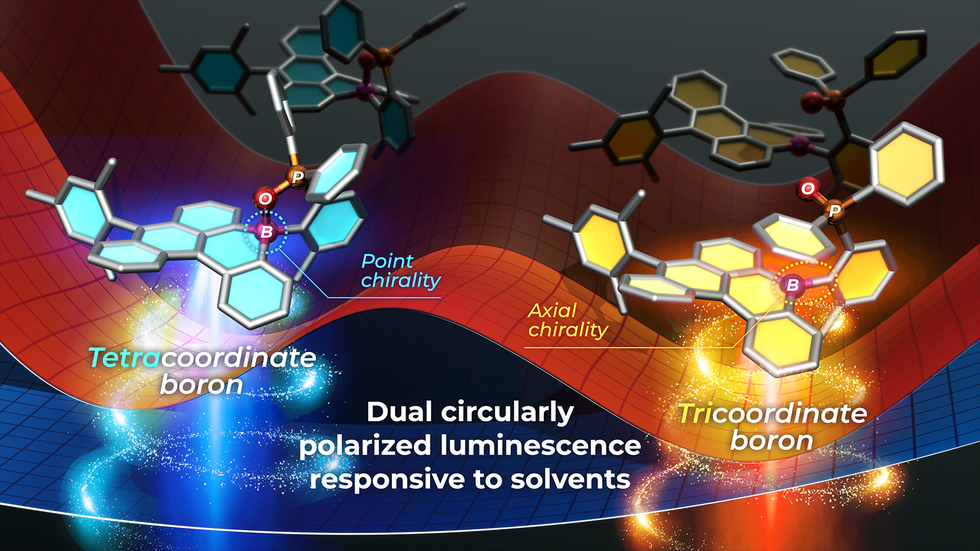
Reversible coordination bonds between boron and heteroatoms endow boron-based π-conjugated frameworks with dynamic functionality, as interconversion between tri- and tetracoordinate boron centers induces pronounced changes in both molecular geometry and electronic structure. In this study, we synthesized a series of chiral boron-embedded polycyclic aromatic hydrocarbons featuring an asymmetric boron center intramolecularly coordinated by a proximal P═O moiety. The resulting tetracoordinate enantiomers exhibit high configurational stability, enabling optical resolution by chiral HPLC. Notably, among the compounds, anthracene-fused boracyclic derivatives display dual circularly polarized luminescence (CPL) arising from excited-state dissociation of the intramolecular P═O⋯B bond, a process whose extent varies depending on the degree of π-extension. In these systems, bond-cleavage converts point chirality in the tetracoordinate state into C−B axial chirality in the tricoordinate state. Taking advantage of the intrinsically superior photophysical properties of tricoordinate boranes, one derivative exhibited CPL with a high quantum yield, while another displayed red-shifted CPL extending to the deep-red region. Furthermore, the dynamic P═O⋯B coordination is highly sensitive to hydrogen-bonding ability and polarity of solvent, thereby giving rise to solvent-dependent dual CPL behavior. These findings establish a new strategy for exploiting reversible coordination in chiral boron-PAHs to access responsive chiroptical functionalities.