2025-09-19 東京科学大学
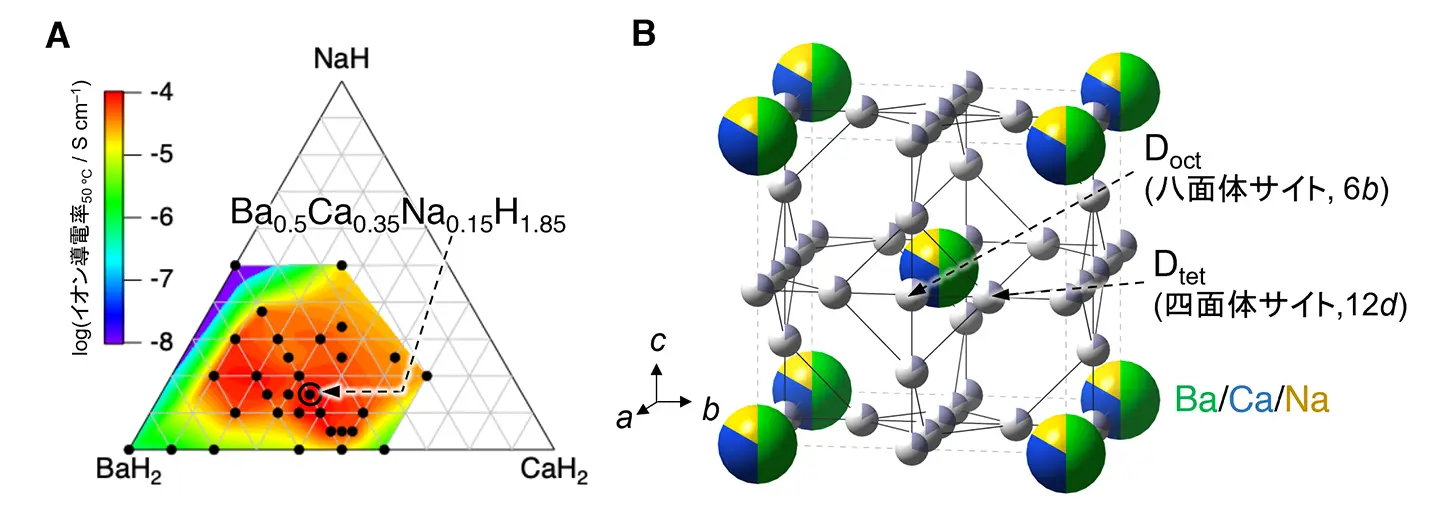
図1. (A)BaH2–CaH2–NaH三成分系のイオン伝導率ヒートマップ。組成式Ba0.5Ca0.35Na0.15H1.85で最も高いイオン伝導率を示した。(B)中性子回折実験により明らかになったBa0.5Ca0.35Na0.15D1.85の結晶構造。Ba/Ca/Naが体心立方格子を形成し、重水素はDM4四面体位置とDM6八面体位置に無秩序に分布する(M = Ba、Ca、Na)。
<関連情報>
- https://www.isct.ac.jp/ja/news/rid72x3ngx43
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=2265&prevId=&key=09c19f5f0c048d9cc796bda6ed284ac1.pdf
- https://www.science.org/doi/10.1126/science.adw1996
H–伝導性固体電解質を用いた高容量・可逆性水素貯蔵 High-capacity, reversible hydrogen storage using H–-conducting solid electrolytes
Takashi Hirose, Naoki Matsui, Takashi Itoh, Yoyo Hinuma, […] , and Ryoji Kanno
Science Published:18 Sep 2025
DOI:https://doi.org/10.1126/science.adw1996
Editor’s summary
Storing hydrogen in the solid state helps to avoid the safety concerns associated with high-pressure gas tanks. However, the widespread application of this method has been limited by the lack of high-performance materials that operate at low temperatures. Hirose et al. explored hydride ion–mediated electrochemical hydrogen storage and identified a promising hydride ion–conducting solid electrolyte from the pseudoternary barium, calcium, sodium hydride system (see the Perspective by O’Hayre and Haile). Its excellent electrochemical stability allows it to be flexibly coupled with various metal-hydride electrodes, and magnesium-hydrogen cells using this electrolyte and a magnesium hydride electrode exhibited a high reversible capacity of 2030 milliampere-hours per gram at a relatively low temperature of 90°C. —Jack Huang
Abstract
Hydrogen absorption and desorption in solids are pivotal reactions involved in batteries and hydrogen storage devices. However, conventional thermodynamic and electrochemical hydrogen storage using high-capacity materials suffers from high hydrogen-desorption temperatures and instability of electrolytes. In this work, we explored electrochemical hydride ion (H–)–driven hydrogen storage and developed a solid electrolyte, anti–α-AgI–type Ba0.5Ca0.35Na0.15H1.85, which exhibits excellent H– conductivity and electrochemical stability. This electrolyte is compatible with several metal-hydrogen electrodes, such as titanim hydride and magnesium hydride (MgH2), allowing for high-capacity, reversible hydrogen storage at low temperatures. Specifically, Mg–H2 cells operating as hydrogen storage devices (Mg + H2 ⇄ MgH2) achieved a reversible capacity of 2030 milliampere hours per gram at 90°C, offering safe and efficient hydrogen-electricity conversion and hydrogen storage devices.