2025-05-20 マサチューセッツ工科大学(MIT)
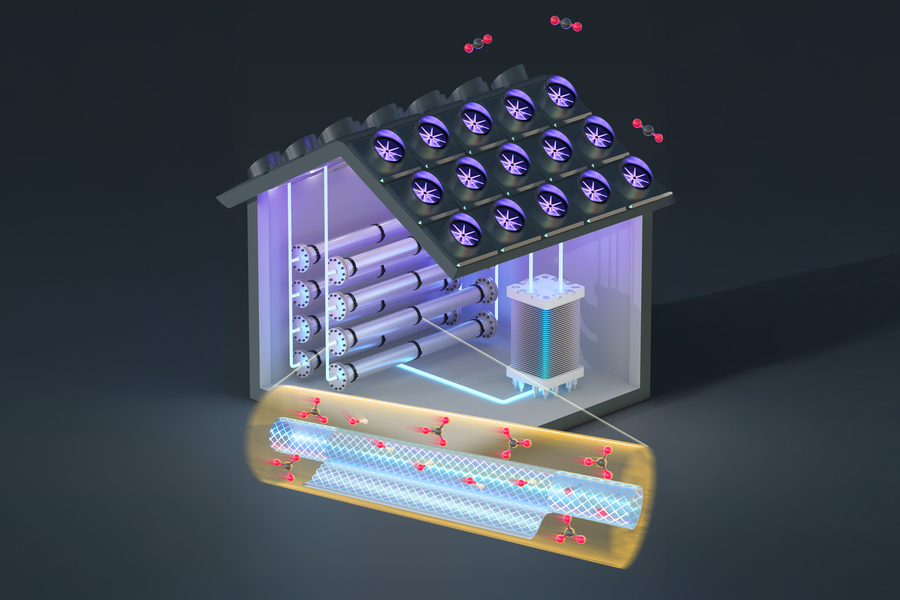
Using nanoscale filtering membranes, researchers at MIT have added a simple intermediate step that makes the process of removing carbon dioxide from the air more efficient. Credits:Image: Courtesy of the researchers
<関連情報>
- https://news.mit.edu/2025/solving-bottleneck-co2-capture-and-conversion-0520
- https://pubs.acs.org/doi/10.1021/acsenergylett.5c00893
炭酸塩/水酸化物分離がCO2吸収速度と電気化学的放出効率を高める Carbonate/Hydroxide Separation Boosts CO2 Absorption Rate and Electrochemical Release Efficiency
Simon Rufer,Tal Joseph,Zara Aamer,and Kripa K. Varanasi
ACS Energy Letters Published May 20, 2025
DOI:https://doi.org/10.1021/acsenergylett.5c00893
Abstract
Electrochemical CO2 capture systems using hydroxide solutions face stiff performance trade-offs, as the hydroxide ions necessary for rapid CO2 absorption reduce the current efficiency of subsequent electrochemical CO2 release. In this work, we propose a carbonate/hydroxide separation step between CO2 absorption and release to provide a concentrated carbonate stream for efficient electrochemical release and a separate hydroxide stream for rapid absorption. We combine experiments on CO2 absorption, nanofiltration separation, and electrochemical release to build a comprehensive model that illustrates system performance trade-offs. We find that employing commercial nanofiltration membranes for separation increases the electrochemical current efficiency by as much as six-fold without sacrificing absorption rate. In the case of Direct Air Capture, the nanofiltration approach reduces costs by 20-30% and significantly increases the operational flexibility of the system. Such carbonate/hydroxide separations may also find use in other systems such as point source capture and integrated CO2 capture and conversion to valuable products.