2024-12-02 バース大学
<関連情報>
- https://www.bath.ac.uk/announcements/controlling-matter-at-the-atomic-level-university-of-bath-breakthrough/
- https://www.nature.com/articles/s41467-024-54677-1
単一分子反応の競合結果を測定することで、古典的アレニウス化学反応速度論が明らかになる Measuring competing outcomes of a single-molecule reaction reveals classical Arrhenius chemical kinetics
Pieter J. Keenan,Rebecca M. Purkiss,Tillmann Klamroth,Peter A. Sloan & Kristina R. Rusimova
Nature Communications Published:28 November 2024
DOI:https://doi.org/10.1038/s41467-024-54677-1
Abstract
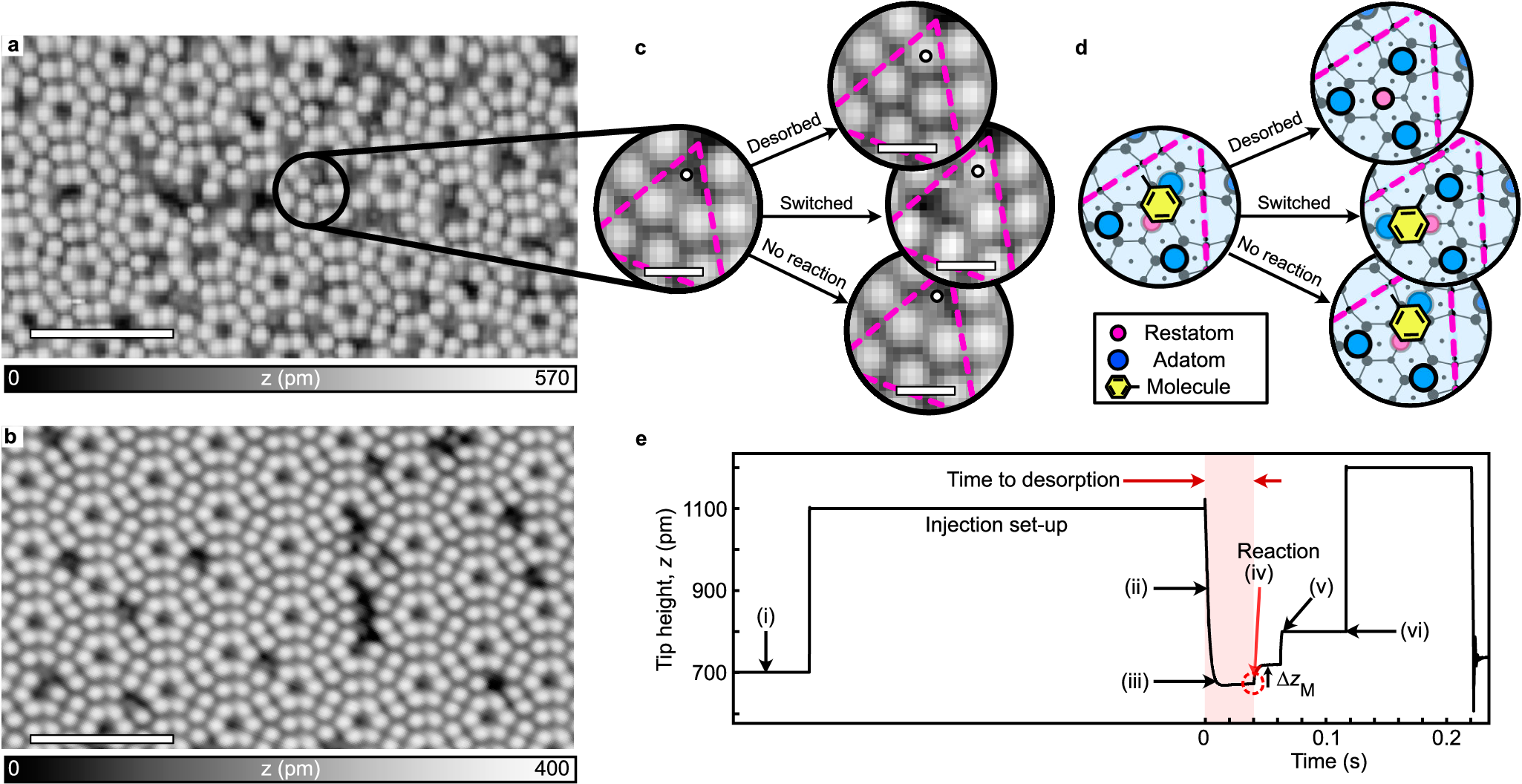
Programming matter one molecule at a time is a long-standing goal in nanoscience. The atomic resolution of a scanning tunnelling microscope (STM) can give control over the probability of inducing single-outcome single-molecule reactions. Here we show it is possible to measure and influence the outcome of a single-molecule reaction with multiple competing outcomes. By precise injection of electrons from an STM tip, toluene molecules are induced to react with two outcomes: switching to an adjacent site or desorption. Within a voltage range set by the electronic structure of the molecule-surface system, we see that the branching ratio between these two outcomes is dependent on the excess energy the exciting electron carries. Using known values, ab initio DFT calculations and empirical models, we conclude that this excess energy leads to a heating of a common intermediate physisorbed state and gives control over the two outcomes via their energy barriers and prefactors.



