2024-05-22 オークリッジ国立研究所(ORNL)
<関連情報>
- https://www.ornl.gov/news/promethium-bound-rare-earth-elements-secrets-exposed
- https://www.nature.com/articles/s41586-024-07267-6
溶液中のプロメチウム錯体の観察 Observation of a promethium complex in solution
Darren M. Driscoll,Frankie D. White,Subhamay Pramanik,Jeffrey D. Einkauf,Bruce Ravel,Dmytro Bykov,Santanu Roy,Richard T. Mayes,Lætitia H. Delmau,Samantha K. Cary,Thomas Dyke,April Miller,Matt Silveira,Shelley M. VanCleve,Sandra M. Davern,Santa Jansone-Popova,Ilja Popovs & Alexander S. Ivanov
Nature Published:22 May 2024
DOI:https://doi.org/10.1038/s41586-024-07267-6
Abstract
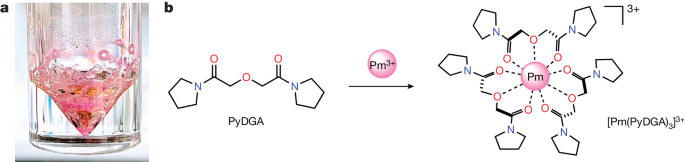
Lanthanide rare-earth metals are ubiquitous in modern technologies1,2,3,4,5, but we know little about chemistry of the 61st element, promethium (Pm)6, a lanthanide that is highly radioactive and inaccessible. Despite its importance7,8, Pm has been conspicuously absent from the experimental studies of lanthanides, impeding our full comprehension of the so-called lanthanide contraction phenomenon: a fundamental aspect of the periodic table that is quoted in general chemistry textbooks. Here we demonstrate a stable chelation of the 147Pm radionuclide (half-life of 2.62 years) in aqueous solution by the newly synthesized organic diglycolamide ligand. The resulting homoleptic PmIII complex is studied using synchrotron X-ray absorption spectroscopy and quantum chemical calculations to establish the coordination structure and a bond distance of promethium. These fundamental insights allow a complete structural investigation of a full set of isostructural lanthanide complexes, ultimately capturing the lanthanide contraction in solution solely on the basis of experimental observations. Our results show accelerated shortening of bonds at the beginning of the lanthanide series, which can be correlated to the separation trends shown by diglycolamides9,10,11. The characterization of the radioactive PmIII complex in an aqueous environment deepens our understanding of intra-lanthanide behaviour12,13,14,15 and the chemistry and separation of the f-block elements16.



