2023-05-30 アルゴンヌ国立研究所(ANL)
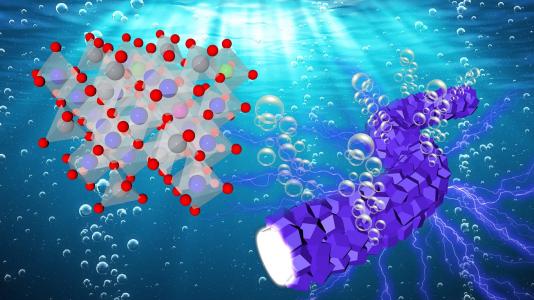
Oxygen bubbles evolving from fibrous, interconnected catalyst particles (right) during electrocatalytic reaction with water. Lattice structure for cobalt-based catalyst on left. (Image by Argonne National Laboratory/Lina Chong and Longsheng Wu using a Shutterstock background.)
◆この新しい触媒はイリジウムよりも安価なコバルトを主成分としており、クリーンな水素の生産コストを大幅に削減することができます。この研究は、クリーンな水素の生産コストを10年以内に1キログラムあたり1ドルまで低減することを目指すアメリカエネルギー省の「Hydrogen Energy Earthshot」イニシアチブに向けた重要な一歩となります。
<関連情報>
- https://www.anl.gov/article/extracting-a-clean-fuel-from-water
- https://www.science.org/doi/10.1126/science.ade1499
プロトン交換膜電解のためのLaおよびMnドープコバルトスピネル酸素発生触媒 La- and Mn-doped cobalt spinel oxygen evolution catalyst for proton exchange membrane electrolysis
Lina Chong,Guoping Gao,Jianguo Wen,Haixia Li,Haiping Xu,Zach Green,Joshua D. Sugar,A. Jeremy Kropf,Wenqian Xu,Xiao-Min Lin,Hui Xu,Lin-Wang Wang and Di-Jia Liu
science Published:11 May 2023
Editor’s summary
Water electrolysis is a potentially sustainable means of producing hydrogen. Unfortunately, only rare and expensive iridium is a sufficiently stable and active oxidation catalyst in the most efficient acidic environment. Chong et al. now report that doping an Earth-abundant cobalt oxide catalyst with lanthanum and manganese ions promotes activity and stability in an acidic proton-exchange membrane water electrolyzer. Simulations suggest that the lanthanum stabilizes the surface of the catalyst and the manganese enhances conductivity in the bulk. —Jake Yeston
Abstract
Discovery of earth-abundant electrocatalysts to replace iridium for the oxygen evolution reaction (OER) in a proton exchange membrane water electrolyzer (PEMWE) represents a critical step in reducing the cost for green hydrogen production. We report a nanofibrous cobalt spinel catalyst codoped with lanthanum (La) and manganese (Mn) prepared from a zeolitic imidazolate framework embedded in electrospun polymer fiber. The catalyst demonstrated a low overpotential of 353 millivolts at 10 milliamperes per square centimeter and a low degradation for OER over 360 hours in acidic electrolyte. A PEMWE containing this catalyst at the anode demonstrated a current density of 2000 milliamperes per square centimeter at 2.47 volts (Nafion 115 membrane) or 4000 milliamperes per square centimeter at 3.00 volts (Nafion 212 membrane) and low degradation in an accelerated stress test.