2023-02-08 オークリッジ国立研究所(ORNL)
◆その結果、水素原子が分子の中心部を高速で移動し、付着している鉄原子の電子のエネルギー準位が大きく変動することを発見した。また、その移動速度は、分子の周囲のわずかな変化で制御でき、驚くべき感度を示した。
◆イェール大学のパトリック・ホランド教授(化学)は、「これは、水素を使って鉄のダイナミクスと電子構造を調整できることを示唆しており、これを利用して化学反応を起こしたり改良したりできる可能性があります」と語っている。
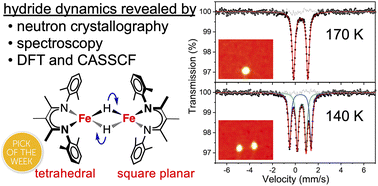
◆水素原子の正確な位置を特定するために、ORNLの核破砕中性子源での中性子回折実験が用いられた。このデータは、新しい触媒の設計にも役立つだろう。
<関連情報>
- https://www.ornl.gov/news/neutrons-uncover-hydrogens-hidden-role-twisting-iron
- https://pubs.rsc.org/en/content/articlelanding/2023/SC/D2SC06412J
S=3基底状態を持つ鉄(II)二量体の急激な水素化物運動による配位子場の動的効果 Dynamic effects on ligand field from rapid hydride motion in an iron(ii) dimer with an S = 3 ground state
Sean F. McWilliams, Brandon Q. Mercado, K. Cory MacLeod, Majed S. Fataftah, Maxime Tarrago, Xiaoping Wang, Eckhard Bill, Shengfa Ye and Patrick L. Holland
Chemical Science Published:08 Feb 2023
DOI:https://doi.org/10.1039/D2SC06412J
Abstract
Hydride complexes are important in catalysis and in iron–sulfur enzymes like nitrogenase, but the impact of hydride mobility on local iron spin states has been underexplored. We describe studies of a dimeric diiron(II) hydride complex using X-ray and neutron crystallography, Mössbauer spectroscopy, magnetism, DFT, and ab initio calculations, which give insight into the dynamics and the electronic structure brought about by the hydrides. The two iron sites in the dimer have differing square-planar (intermediate-spin) and tetrahedral (high-spin) iron geometries, which are distinguished only by the hydride positions. These are strongly coupled to give an Stotal = 3 ground state with substantial magnetic anisotropy, and the merits of both localized and delocalized spin models are discussed. The dynamic nature of the sites is dependent on crystal packing, as shown by changes during a phase transformation that occurs near 160 K. The change in dynamics of the hydride motion leads to insight into its influence on the electronic structure. The accumulated data indicate that the two sites can trade geometries by rotating the hydrides, at a rate that is rapid above the phase transition temperature but slow below it. This small movement of the hydrides causes large changes in the ligand field because they are strong-field ligands. This suggests that hydrides could be useful in catalysis not only due to their reactivity, but also due to their ability to rapidly modulate the local electronic structure and spin states at metal sites.