2026-01-06 バース大学
<関連情報>
- https://www.bath.ac.uk/announcements/scientists-discover-key-to-solving-an-80-year-old-chemistry-puzzle/
- https://www.nature.com/articles/s41557-025-02022-4
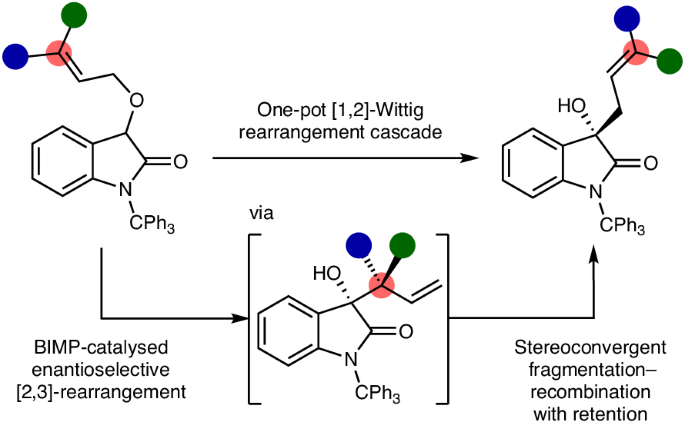
アリルエーテルの触媒的エナンチオ選択的[1,2]-ウィッティヒ転位カスケード The catalytic enantioselective [1,2]-Wittig rearrangement cascade of allylic ethers
Tengfei Kang,Justin O’Yang,Kevin Kasten,Samuel S. Allsop,Toby Lewis-Atwell,Elliot H. E. Farrar,Martin Juhl,David B. Cordes,Aidan P. McKay,Matthew N. Grayson & Andrew D. Smith
Nature Chemistry Published:06 January 2026
DOI:https://doi.org/10.1038/s41557-025-02022-4
Abstract
The catalytic enantioselective [1,2]-Wittig rearrangement of allylic ethers constitutes a recognized synthetic challenge as it is traditionally considered to arise from a non-concerted reaction pathway via formation and recombination of radical pairs. Here we show a catalytic enantioselective solution to this challenge, demonstrating that [1,2]-Wittig products are generated via an alternative reaction cascade to traditional dogma. The developed process employs a chiral bifunctional iminophosphorane catalyst to promote an initial enantioselective [2,3]-sigmatropic rearrangement. A subsequent base-promoted, stereoconvergent, fragmentation–recombination process that proceeds with high enantiospecificity and retention of configuration, formally equivalent to a Woodward–Hoffmann forbidden thermal [1,3]-sigmatropic rearrangement, generates [1,2]-Wittig products in up to 97:3 enantiomeric ratio. Supported by extensive quantum chemistry calculations, this chirality transfer process will have broad implications for fundamental stereocontrol in organic transformations.