2025-11-28 大阪大学,科学技術振興機構
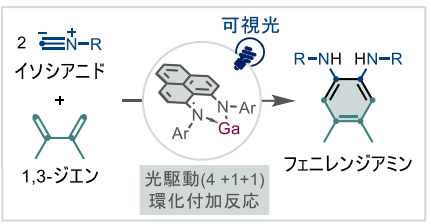
図 ガリウム(Ga)の(I/III)レドックス反応を利用した (4+1+1)光環化付加型フェニレンジアミン合成
<関連情報>
- https://www.jst.go.jp/pr/announce/20251128/index.html
- https://www.jst.go.jp/pr/announce/20251128/pdf/20251128.pdf
- https://pubs.acs.org/doi/10.1021/jacs.5c15802
ガリウム(I)/(III)酸化還元を利用した1,3-ジエンとイソシアニドの(4 + 1 + 1)光環化付加によるフェニレンジアミンの合成:フェナレニル系配位子の重要な役割 Synthesis of Phenylenediamines via (4 + 1 + 1) Photocycloaddition of 1,3-Dienes and Isocyanides Enabled by a Gallium(I)/(III) Redox: The Key Role of a Phenalenyl-Based Ligand
Nijito Mukai,Takuya Kodama,and Mamoru Tobisu
Journal of the American Chemical Society Published: November 28, 2025
DOI:https://doi.org/10.1021/jacs.5c15802
Abstract
A gallacyclopentene complex is generated via (4 + 1) cycloaddition of a Ga(I) complex bearing a phenalenyl-based ligand with a 1,3-diene. Upon visible-light irradiation, the Ga(III) complex undergoes homolytic cleavage of a Ga–C bond, facilitating the subsequent mono- and double-insertion reactions with isocyanides. The double insertion complex undergoes C–C bond formation to afford 1,2-phenylenediamine, achieving a (4 + 1 + 1) cycloaddition of 1,3-dienes and isocyanides via a stoichiometric redox process and insertion on gallium. This study demonstrates a proof-of-concept for the formal two-electron redox processes of group 13 elements for organic synthesis enabled by the involvement of radical intermediates.