2025-09-24 パシフィック・ノースウェスト国立研究所(PNNL)
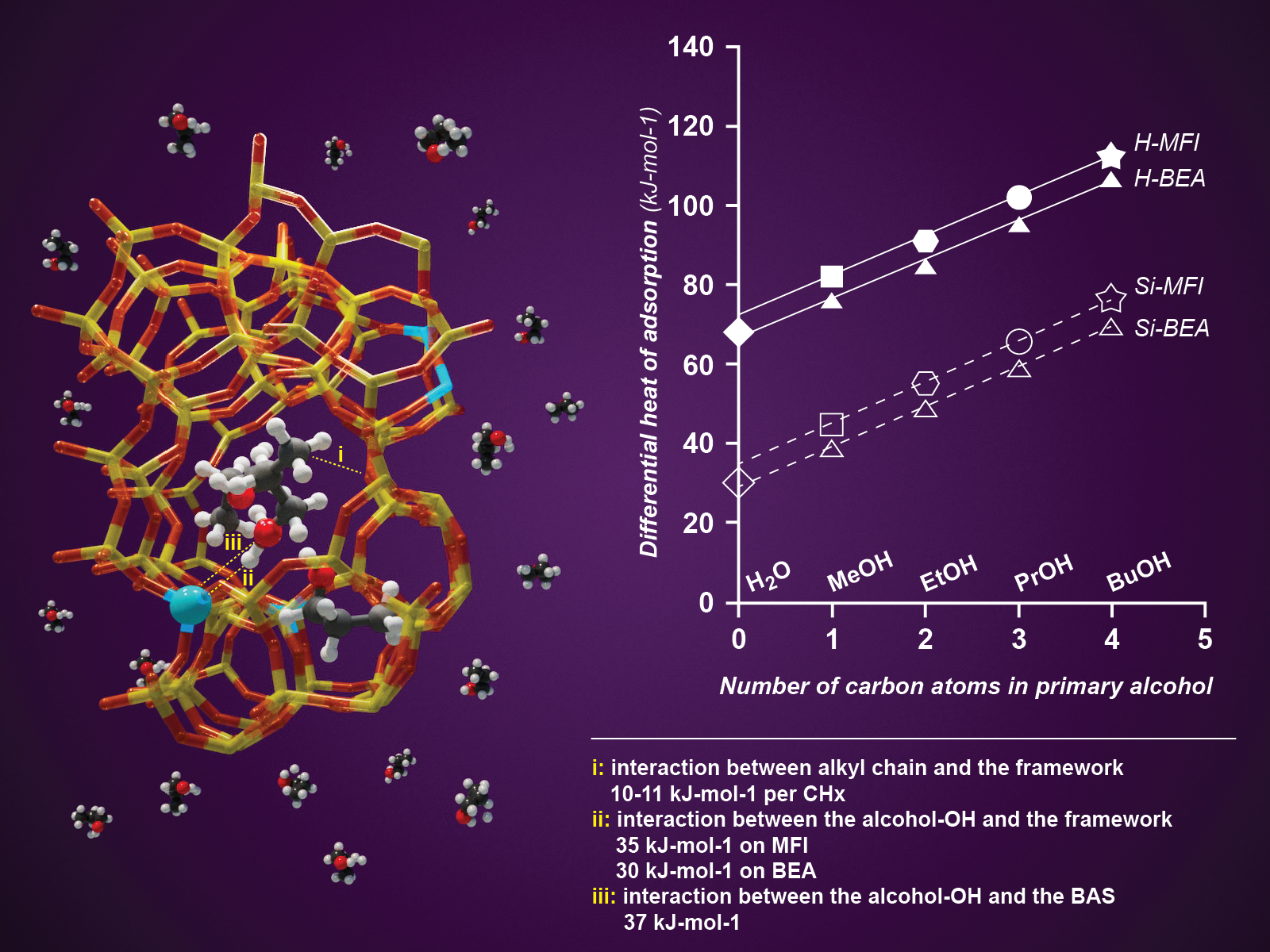
Researchers quantified three main interaction types between short chain alcohols and zeolite pore walls.
(Image by Cortland Johnson | Pacific Northwest National Laboratory)
<関連情報>
- https://www.pnnl.gov/publications/quantifying-molecular-interactions-alcohols-zeolite-pores
- https://pubs.acs.org/doi/10.1021/jacs.5c09340
ゼオライト細孔内におけるアルコールの極性基と非極性基の相互作用 Interactions of Polar and Nonpolar Groups of Alcohols in Zeolite Pores
Ruixue Zhao,Sungmin Kim,Mal-Soon Lee,Benjamin A. Jackson,Fuli Deng,Xiaomai Chen,Cong Zhou,Konstantin Khivantsev,Yue Liu,Vassiliki-Alexandra Glezakou,Roger Rousseau,and Johannes A. Lercher
Journal of the American Chemical Society Published: July 12, 2025
DOI:https://doi.org/10.1021/jacs.5c09340
Abstract
Understanding the quantitative interactions among zeolite pore walls, Bro̷nsted acid sites, and molecules with both polar and nonpolar regions is essential for scoping out the potential of zeolites as sorbents and catalysts. Purely siliceous zeolites (MFI and Beta in the present study) are hydrophobic, whereas those containing aluminum are considered hydrophilic, preferentially adsorbing organic molecules even in aqueous environments. To characterize these interactions, we use primary alcohols of increasing molecular weight, quantifying their specific interactions in the confined pore space of the alkyl (CHx) and OH groups. Three types of interactions were identified: (i) alkyl CHx groups interacting with the zeolite pore walls (approximately 10 kJ mol–1 per carbon), (ii) alcohol OH groups interacting with the pore walls (30–35 kJ mol–1), and (iii) alcohol OH groups interacting with Bro̷nsted acid sites (37 kJ mol–1). All three interactions were well mirrored by computational simulations. The contribution of the alkyl CHx groups was inferred from the incremental increase in sorption enthalpy with increasing molecular weight; the interaction strength of the OH groups was determined by extrapolating the global adsorption enthalpy of the alcohols to a hypothetical OH group without an alkyl group. This value was identical to the adsorption enthalpy of water. The experiments demonstrated that only water has an adsorption enthalpy on zeolite pore walls lower than its condensation enthalpy (30–35 kJ mol–1 vs 45 kJ mol–1), limiting the concentration of water that can be adsorbed.