2025-06-06 パシフィック・ノースウェスト国立研究所 (PNNL)
![]()
<関連情報>
- https://www.pnnl.gov/publications/advancing-understanding-methyl-coenzyme-m-reductase
- https://pubs.acs.org/doi/full/10.1021/acs.accounts.4c00730
メチル-コエンザイムM還元酵素(MCR)の化学における構造と機構の進歩 Structural and Mechanistic Advances in the Chemistry of Methyl-Coenzyme M Reductase (MCR)
Bojana Ginovska,Simone Raugei,Stephen W. Ragsdale,Christopher Ohmer,Ritimukta Sarangi
Accounts of Chemical Research Published: March 5, 2025
DOI:https://doi.org/10.1021/acs.accounts.4c00730
Abstract
Conspectus
Methane represents 34% of U.S. energy consumption and is a major greenhouse gas related to the global carbon cycle and energy production. However, current industrial practices significantly increase atmospheric methane levels, necessitating a deeper understanding of its biosynthesis and oxidation. Methyl-coenzyme M reductase (MCR) is central to biological methane metabolism, catalyzing the final step of methanogenesis and the first step in anaerobic methane oxidation. It is also a key target for strategies to capture and transform methane into value-added chemicals.
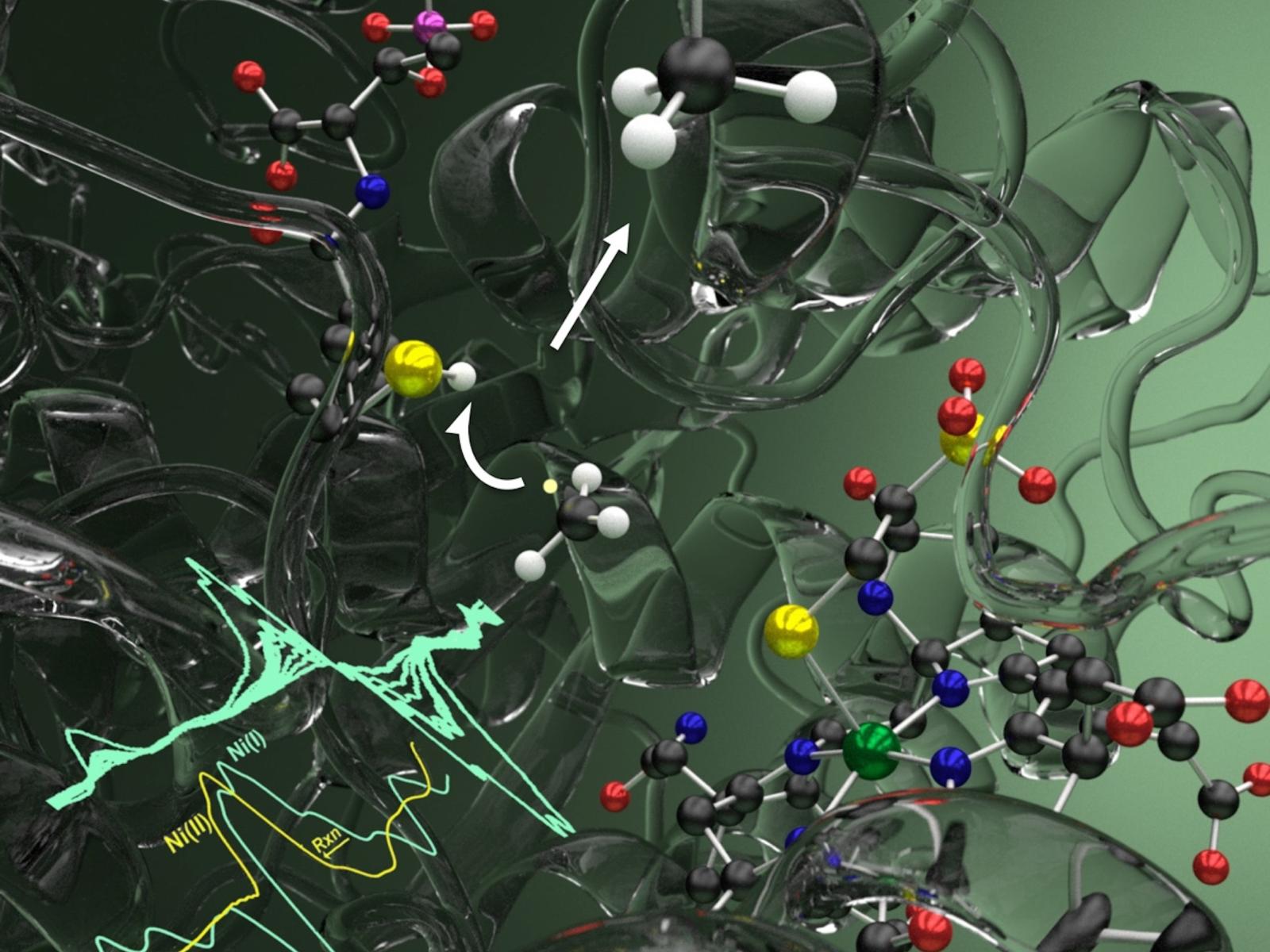
The active site of MCR is a buried Ni-based cofactor only accessible by the substrates via a 50 Å long tunnel. Although the Ni(I) state is required to initiate catalysis, capturing this state remains a challenge for the current structural techniques. Recent advances in structural biology using X-ray Free-Electron Laser serial crystallography have provided insights into MCR’s inactive Ni(II) state at room temperature and show promise for capturing its active Ni(I) form.
Our team has established several critical aspects of the MCR mechanism using a combination of experimental and computational studies. MCR uses CH3–SCoM and CoBSH as substrates, producing methane and a disulfide product CoMSSCoB. Kinetic analysis showed that productive substrate binding requires CH3–SCoM to bind first, inducing conformational changes that optimize the active site for subsequent CoBSH binding. Following substrate binding, four proposed methane production/oxidation mechanisms were examined, establishing whether the reaction proceeds through an organometallic methyl-nickel(III), methyl anion ion, or methyl radical intermediate. Experimental measurements using CoBSH analogs successfully slowed the reaction, allowing for mechanistic insight that demonstrated the methyl radical pathway, where the initial interactions involve homolytic cleavage of the methyl-sulfur bond, generating a methyl radical that quickly abstracts the thiol hydrogen atom of CoBSH to form methane. Computational studies further confirmed that, compared to other mechanisms, the methyl radical mechanism is thermodynamically more favorable and accessible under physiological conditions.
Spectroscopic and computational studies challenged the conventional understanding of substrate binding in MCR by proposing an alternative positioning of CH3–SCoM and CoMSSCoB in the active site pocket. The research suggested that CH3–SCoM (substrate) and CoMSSCoB (product) bind via their sulfonate groups to the Ni(I) center of cofactor F430. This binding allows for the reaction without substrate reorganization in the pocket but would require a long-range electron transfer.
Overall, the work summarized in this review reflects our current understanding of the enzyme’s catalytic mechanism and structural dynamics. This is essential for developing efficient methane conversion technologies that could mitigate its environmental impact while harnessing energy-storage properties.