2025-06-05 東京大学
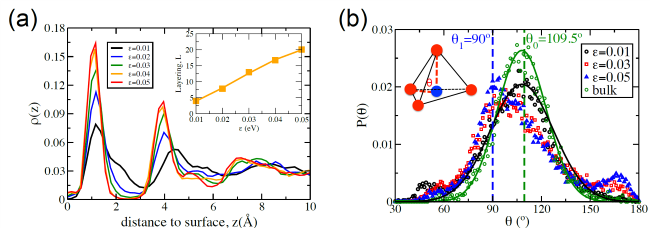
図1: T = 210 K における界面液体の層構造および次元性。
(a) 固体表面近傍における液体水の層構造。氷核生成前、 T = 210 K における液体水の密度分布(相互作用(親水性)強度 ε = 0.01、0.02、0.03、0.04、0.05 eV)。挿入図:基板と接する液体水の層構造の程度 L は、 ε の増加に伴って増加します。(b) 接触層に存在する水分子と、破線で結ばれた2つの隣接分子によって形成される角度 θ の分布(挿入図を参照)。緑と青の破線は、それぞれ主なピーク位置 θ ₀ = 109.5° および θ ₁ = 90° を示します。
<関連情報>
- https://www.iis.u-tokyo.ac.jp/ja/news/4784/
- https://www.sciencedirect.com/science/article/pii/S0021979725012032?via%3Dihub
氷の形成における表面近傍の水の構造が果たす秘密の役割 The secret role of water’s structure near surfaces in ice formation
Gang Sun, Hajime Tanaka
Journal of Colloid and Interface Science Available online: 12 May 2025
DOI:https://doi.org/10.1016/j.jcis.2025.137812
Highlights
- Most ice forms on surfaces, as pure water needs deep supercooling.
- We use simulations to study ice formation on a simple cubic surface.
- Ice nucleation depends on water’s structure, not ice’s surface affinity.
- Ordering in two surface water layers plays a key role in triggering ice nucleation.
- Understanding this can help control ice in nature and technology.
Abstract
Hypothesis
Most ice on Earth forms via heterogeneous nucleation, as homogeneous nucleation requires significant supercooling. Despite its prevalence, the microscopic mechanisms behind this process remain unclear. We hypothesize that ice nucleation is primarily driven by low-dimensional structural preordering in interfacial liquid layers, rather than by the surface’s direct affinity for bulk ice.
Simulations
To test this hypothesis, we perform molecular dynamics simulations of ice nucleation on a simple cubic substrate with tunable hydrophilicity. We analyze layering, hydrogen-bond distortions, orientational order, and substrate-ice lattice matching to uncover the physical mechanisms that control nucleation pathways.
Findings
Bilayer hexagonal ice forms on all substrates, but the nucleation pathway depends sensitively on surface hydrophilicity. At low hydrophilicity, bylayer ice nucleates directly from interfacial water. As hydrophilicity increases, enhanced planarity and density promote sequential nucleation, with two-dimensional ice forming first in the contact layer, then in the second layer. Excessive hydrophilicity hinders this process by suppressing 2D ordering in the contact layer, reversing the nucleation sequence. Consequently, the nucleation rate is maximized at intermediate hydrophilicity. Furthermore, we find that crystalline preordering in the contact layer is strongest when the substrate lattice closely matches that of ice, minimizing the free energy barrier for nucleation. These results highlight how surface-induced liquid ordering — rather than simple templating — controls ice formation. This mechanism likely extends to tetrahedral liquids such as silicon, germanium, carbon, and silica, underscoring the universal role of interfacial liquid structuring in surface-assisted crystallization across natural and technological systems.



