2025-04-15 パシフィック・ノースウェスト国立研究所(PNNL)
<関連情報>
- https://www.pnnl.gov/news-media/discovery-opens-doors-cheaper-and-quicker-battery-manufacturing
- https://www.nature.com/articles/s41560-025-01738-4
Li2Oの特異な昇華が単結晶成長と焼結を促進する Unusual Li2O sublimation promotes single-crystal growth and sintering
Bingbin Wu,Ran Yi,Yaobin Xu,Peiyuan Gao,Yujing Bi,Libor Novák,Zhao Liu,Enyuan Hu,Nan Wang,Job Rijssenbeek,Subramanian Venkatachalam,Jing Wu,Dianying Liu,Xia Cao & Jie Xiao
Nature Energy Published:06 March 2025
DOI:https://doi.org/10.1038/s41560-025-01738-4
Abstract
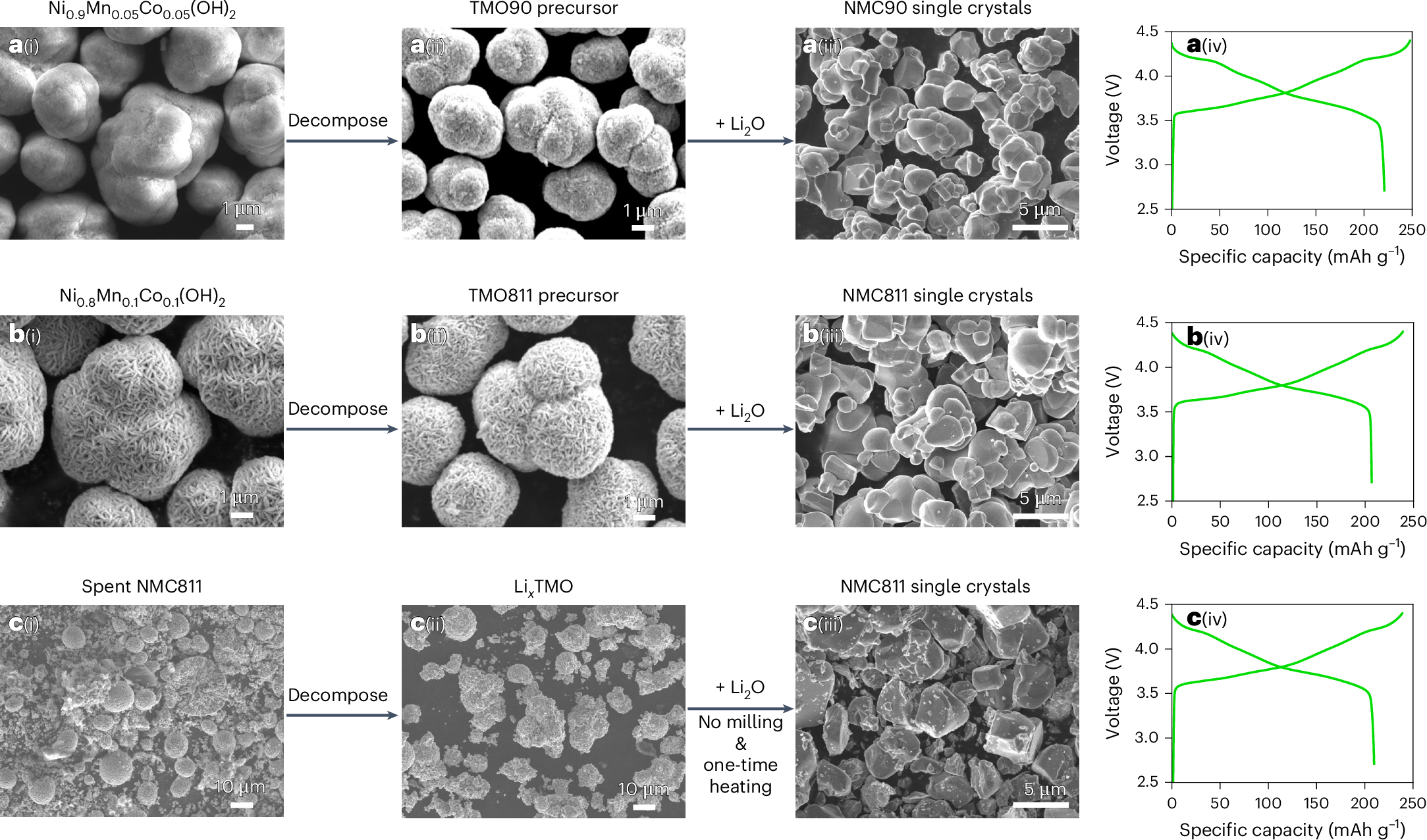
Li2O is rarely used for cathode material synthesis due to its high melting point (1,438 °C). Here we discover that Li2O can sublimate at 800–1,000 °C under ambient pressure, opening new possibilities for cathode synthesis. We propose a mechanism that enables synthesis of single crystals—such as LiNi0.8Mn0.1Co0.1O2 (NMC811) or LiNi0.9Mn0.05Co0.05O2 (NMC90)—without direct contact with Li2O salts. We show that Li2O vapour successfully converts spent polycrystalline NMC811 into segregated single crystals without milling or post-treatment. The Li2O vapour, derived from Li2O solids, diffuses rapidly and reacts with precursors, mimicking a molten-salt environment, which facilitates single-crystal growth. The chemical lithiation process continuously drives Li2O sublimation, sintering the crystals. Single crystals derived from Li2O and fresh precursors or spent polycrystals exhibit outstanding cycling after 1,000 cycles in full cells. The demonstrated Li2O sublimation and its universal role in promoting single-crystal growth provides an effective approach for single-crystal synthesis, scale-up and recycling.



