2025-03-24 中国科学院(CAS)
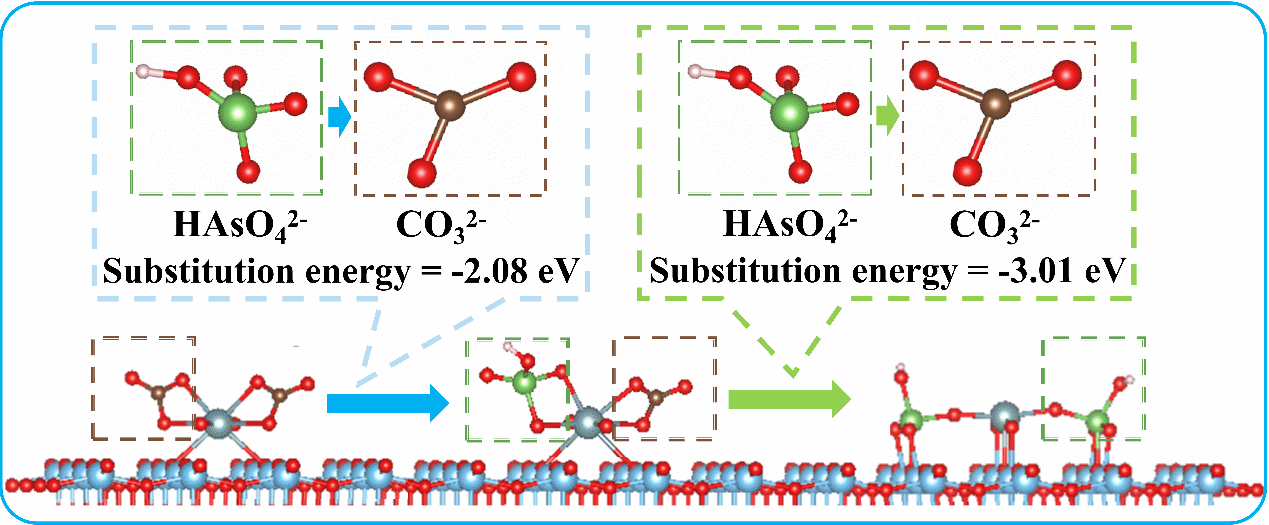 Formation Mechanism of the Ternary Complex [Ti–U(VI)–As(V)] (Image by LI Zheng)
Formation Mechanism of the Ternary Complex [Ti–U(VI)–As(V)] (Image by LI Zheng)
<関連情報>
- https://english.cas.cn/newsroom/research_news/earth/202503/t20250324_908642.shtml
- https://www.pnas.org/doi/10.1073/pnas.2501354122
酸化チタンによるヒ素とウランの同時除去を促進する三元表面錯体形成 Ternary surface complexation promotes simultaneous removal of arsenic and uranium by TiO2
Leiming Lin, Zheng Li, Jun Ren, +1 , and Fubo Luan
Proceedings of the National Academy of Sciences Published:March 20, 2025
DOI:https://doi.org/10.1073/pnas.2501354122
Significance
Groundwater containing naturally occurring arsenic and uranium is widely used for drinking purpose in numerous countries, presenting a significant public health concern. However, there is a lack of economically efficient technology for the simultaneous removal of arsenic and uranium. Our research uncovered a mechanism involving the formation of a ternary surface complex [Ti–U(VI)–As(V)] on the TiO2 surface confirmed through density functional theory calculations. This provides valuable insights into the adsorption processes of arsenic and uranium, contributing to the development of strategies for their simultaneous removal. Our study offers a promising solution for addressing the simultaneous removal of arsenic and uranium from groundwater, thereby reducing the human health risks associated with using this groundwater as a drinking water source.
Abstract
The co-occurrence of arsenic and uranium in groundwater has been found in many countries, posing a significant challenge to human health. Here, we have demonstrated the efficient simultaneous removal of arsenic and uranyl-carbonate complexes from groundwater using {001}-TiO2. Surprisingly, the presence of U(VI) greatly enhanced the adsorption of As(V) on {001}-TiO2, while As(V) had a negligible impact on U(VI) adsorption. Through in situ ATR-FTIR spectroscopy, we uncovered a mechanism involving the formation of a ternary surface complex [Ti–U(VI)–As(V)] on the surface of {001}-TiO2. This ternary surface complex formed through the substitution of CO32- from uranyl coordination sites. Furthermore, the adsorbed As(V) and U(VI) can be easily recovered using a sodium hydroxide solution, and {001}-TiO2 can be used repeatedly. Our findings offer a promising solution for the simultaneous removal of As(V) and U(VI) from groundwater and provide valuable insights into the mechanisms involved in their removal.