2024-04-25 ジョージア工科大学
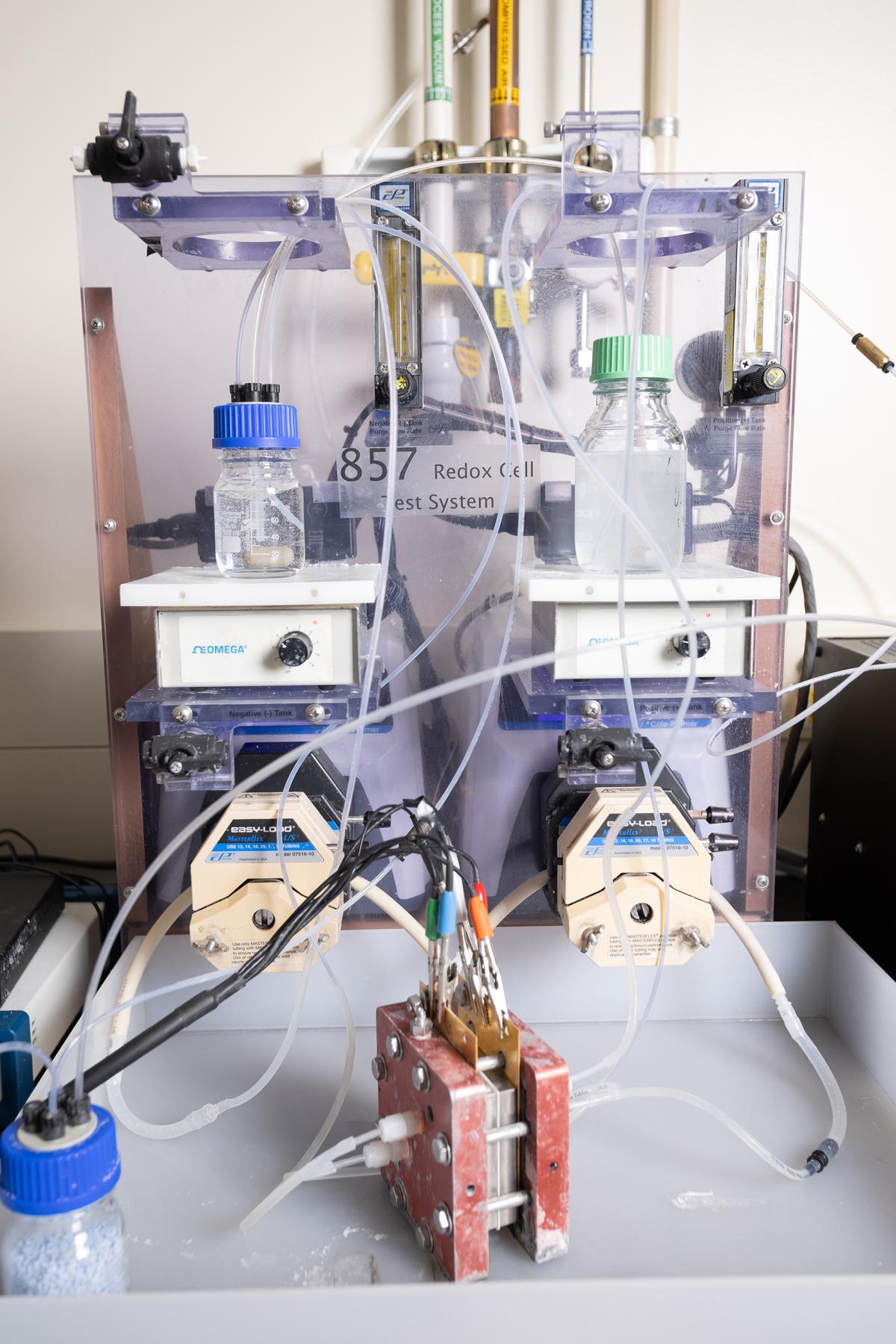
The experimental setup researchers in Marta Hatzell’s lab used to test their new electrochemical reactor for carbon capture. (Photo: Candler Hobbs)
<関連情報>
- https://coe.gatech.edu/news/2024/04/new-approach-could-make-reusing-captured-carbon-far-cheaper-less-energy-intensive
- https://pubs.rsc.org/en/Content/ArticleLanding/2024/EE/D4EE00048J
バイポーラ膜電解による重炭酸塩からの統合的炭素回収とCO生産 Integrated carbon capture and CO production from bicarbonates through bipolar membrane electrolysis
Hakhyeon Song, Carlos A. Fernandez, Hyeonuk Choi, Po-Wei Huang, Jihun Oh and Marta C. Hatzell
Energy & Environmental Science published:16 Apr 2024
DOI:https://doi.org/10.1039/D4EE00048J
Abstract
Electrochemical CO2 reduction (CO2RR) offers an environmentally friendly method to transform and harness sequestered CO2. While gas-phase electrolysis systems provide high efficiency, gas-phase electrolysis systems face challenges related to carbonate precipitate formation and crossover. In response, liquid-phase (bi)carbonates electrolysis systems based on the use of bipolar membrane (BPM) electrode assemblies have emerged. These systems not only streamline the carbon-capture and conversion process but also present economic benefits. However, liquid-phase (bi)carbonate electrolysis cells suffer limited stability and selectivity at relevant operating current. Here, utilizing a Ni-based single-atom catalyst (Ni-SAC) and bicarbonate electrolyte, we demonstrate exceptional CO Faradaic efficiency (93%) at a partial current density of -186 mA cm-2 at -3.7 V for over 18 hours with an integrated carbon capture system. Furthermore, we conducted a comparative analysis of various performance and economic metrics between bicarbonate electrolysis and CO2 gas electrolysis. Our results highlight the superior advantages of BPM-based electrolysis in terms of CO2 utilization efficiency, stability, and CO product concentration in the outlet stream. Despite its higher energy demand in BPM-based electrolysis, this approach presents a technologically promising alternative to conventional CO2 gas-phase systems. This breakthrough paves the way for the efficient direct carbon capture and conversion, offering a promising pathway towards a more sustainable and carbon-neutral future.