2023-04-20 ワシントン大学セントルイス校
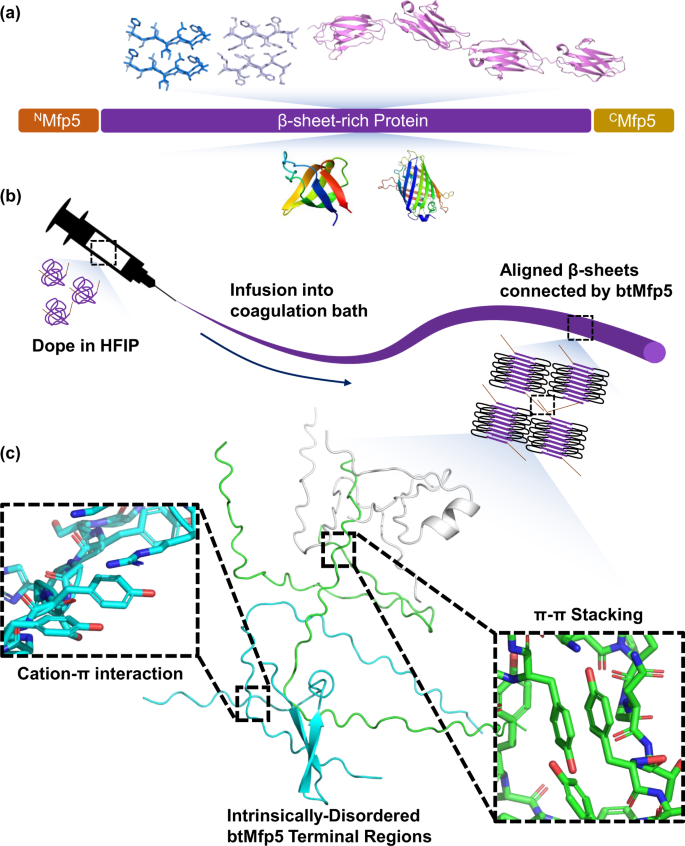
天然変性ムール貝足タンパク質断片を末端に融合させ、エンドツーエンドのタンパク質間相互作用を促進することにより、
この方法は、ナイロンやポリエステルなどの石油由来の繊維素材に代わる再生可能で生分解性の高い素材となり、従来の繊維素材よりも環境に優しいため、衣料品製造に革命をもたらすことが期待されます。
新しいスパイダーシルク融合タンパク質は、組み換えシルクタンパク質よりも8倍高い収率を示し、btMSilk繊維は軽量でありながら強度と強靭性が大幅に向上しています。この研究成果は、4月14日付の『Nature Communications』に掲載されました。
<関連情報>
- https://source.wustl.edu/2023/04/synthetic-biology-meets-fashion-in-engineered-silk/
- https://engineering.wustl.edu/news/2023/Synthetic-biology-meets-fashion-in-engineered-silk.html
- https://www.nature.com/articles/s41467-023-37563-0
合成タンパク質の繊維の強度を向上させる、天然変性ムール貝の足タンパク質断片を両末端に融合 Bi-terminal fusion of intrinsically-disordered mussel foot protein fragments boosts mechanical strength for protein fibers
Jingyao Li,Bojing Jiang,Xinyuan Chang,Han Yu,Yichao Han & Fuzhong Zhang
Nature Communications Published:14 April 2023
DOI:https://doi.org/10.1038/s41467-023-37563-0
Abstract
Microbially-synthesized protein-based materials are attractive replacements for petroleum-derived synthetic polymers. However, the high molecular weight, high repetitiveness, and highly-biased amino acid composition of high-performance protein-based materials have restricted their production and widespread use. Here we present a general strategy for enhancing both strength and toughness of low-molecular-weight protein-based materials by fusing intrinsically-disordered mussel foot protein fragments to their termini, thereby promoting end-to-end protein-protein interactions. We demonstrate that fibers of a ~60 kDa bi-terminally fused amyloid-silk protein exhibit ultimate tensile strength up to 481 ± 31 MPa and toughness of 179 ± 39 MJ*m−3, while achieving a high titer of 8.0 ± 0.70 g/L by bioreactor production. We show that bi-terminal fusion of Mfp5 fragments significantly enhances the alignment of β-nanocrystals, and intermolecular interactions are promoted by cation-π and π-π interactions between terminal fragments. Our approach highlights the advantage of self-interacting intrinsically-disordered proteins in enhancing material mechanical properties and can be applied to a wide range of protein-based materials.