2026-01-14 中国科学院(CAS)
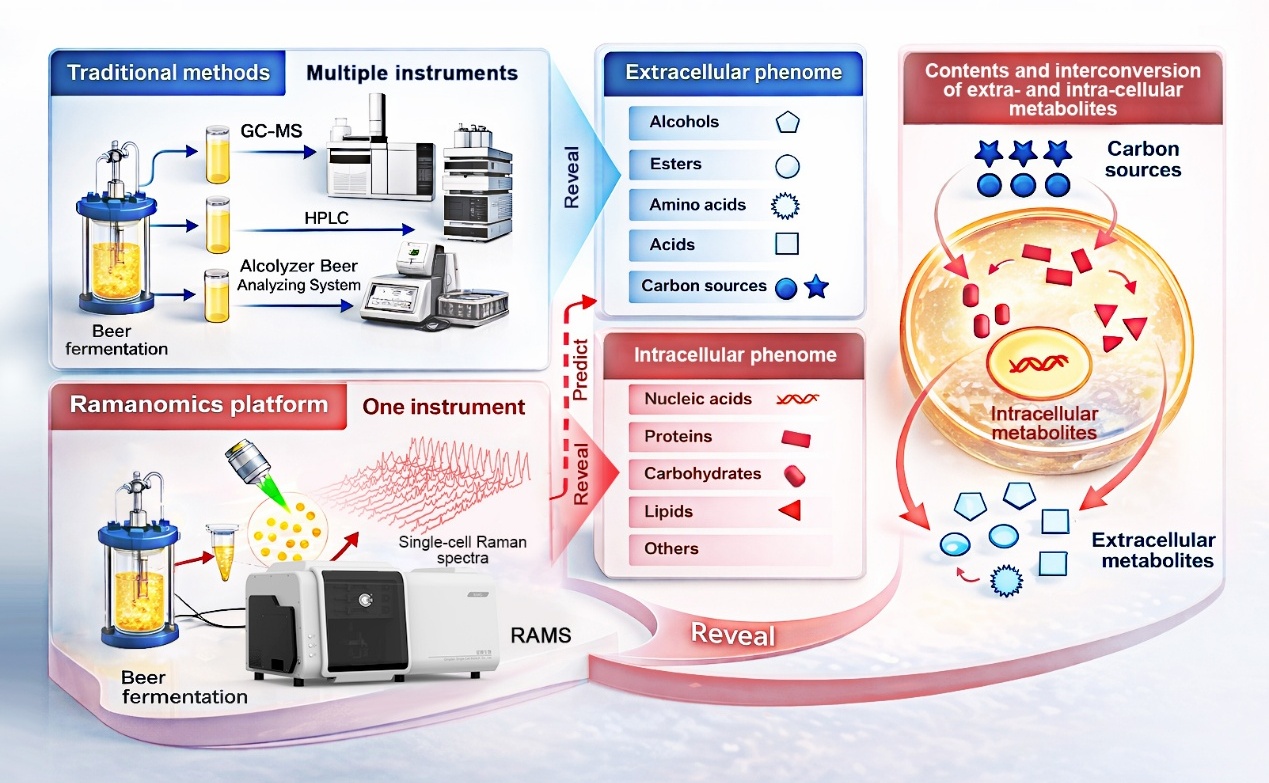
Overview of “process ramanomics”. Single-cell Raman fingerprints collected across fermentation provide a fast, label-free window into brewing progress with single-cell resolution. (Image by LIU Yang)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202601/t20260114_1145714.shtml
- https://www.sciencedirect.com/science/article/pii/S0960852425017559
ビール発酵中の細胞外および細胞内代謝産物の生成と相互変換をラマノミクスで追跡 Tracking production and interconversion of extra- and intra-cellular metabolites during beer fermentation by ramanomics
Yang He, Yuehui He, Yuanyuan Zhou, Xunrong Li, Xinran Zhang, Yuetong Ji, Junhong Yu, Jian Xu
Bioresource Technology Available online: 9 December 2025
DOI:https://doi.org/10.1016/j.biortech.2025.133788
Highlights
- Single-Cell Raman Spectrum (SCRS) enables measurement of intracellular metabolites in individual beer yeast cells.
- The contents of extracellular metabolites of individual beer yeast can be modelled at single-cell resolution by SCRS.
- Ramanome analysis facilitates real-time monitoring of population heterogeneity in beer yeast during fermentation.
- Intra-Ramanome Correlation Analysis (IRCA) reveals potential interconversions of extra- and intra-cellular metabolites.
Abstract
Cellular metabolic state and its heterogeneity are pivotal features that determine fermentation productivity, yet label-free monitoring has generally been difficult. Employing beer fermentation by Saccharomyces pastorianus as a model, we demonstrated that temporal sampling of ramanomes, the collection of spontaneous Single-Cell Raman Spectra (SCRS) from an isogenic population, provides rich insights into the profiles and inter-conversion of both intra- and extra-cellular metabolites. Among 43 extracellular metabolic phenotypes, ramanomes successfully modeled 19 of them, including the extracellular levels of four alcohols, four esters, four amino acids, two acids, and four mono- and di-saccharide substrates, plus the alcohol-to-ester ratio. Moreover, Intra-Ramanome Correlation Analysis (IRCA) revealed potential metabolic interactions in pairs of intracellular metabolites, extracellular metabolites, and medium substrates. Specifically, carbohydrates were the most active intracellular metabolites, while proteins significantly influenced alcohol and ester synthesis on Day 1 of fermentation. Additionally, both alcohols and esters showed negative correlations with extracellular amino acids and acids. The global-IRCN average degree, reflecting metabolic network complexity, increased over time and was positively correlated with extracellular levels of key products such as n-propanol and various esters, while negatively correlated with acetic acid and certain sugars. Therefore, by enabling non-destructive, label-free, and rapid modeling of both intra- and extracellular metabolite levels, ramanomics can find wide applications in process monitoring and control.