2025-05-09 ワシントン大学セントルイス校
<関連情報>
- https://source.washu.edu/2025/05/the-mysterious-chemical-world-inside-nanopores/
- https://engineering.washu.edu/news/2025/The-mysterious-chemical-world-inside-nanopores.html
- https://pubs.acs.org/doi/10.1021/acsami.4c15940
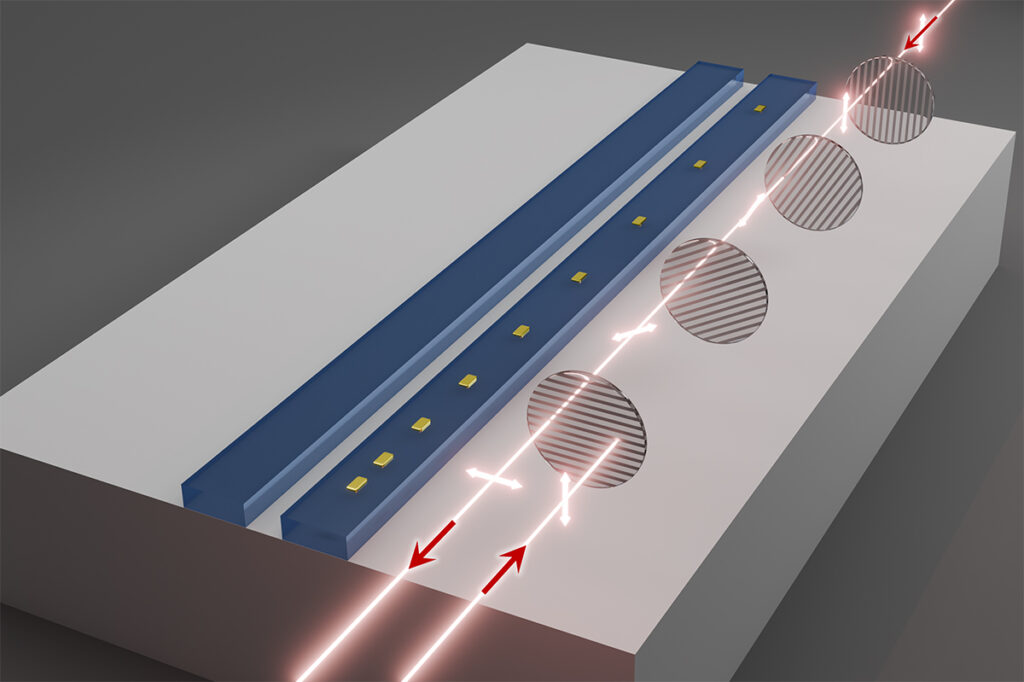
化学官能基がナノ細孔内のイオン濃度とpHを制御する Chemical Functional Groups Regulate Ion Concentrations and pHs in Nanopores
Yaguang Zhu,Prashant Gupta,Hamed Gholami Derami,Yin-Yuan Huang,Srikanth Singamaneni,and Young-Shin Jun
ACS Applied Materials & Interfaces Published: April 29, 2025
DOI:https://doi.org/10.1021/acsami.4c15940
Abstract

Understanding ion behaviors in functionalized nanopores is essential to deciphering reactions in both natural and engineered systems, such as sediments, biological ion channels, and membranes. While many efforts have shown the modified ion behaviors in the functionalized nanopores, a direct measurement and analysis to show how chemical functional groups affect ion concentrations in nanopores are critically needed. Herein, we present a plasmonic nanosensor that can measure the local concentrations of protons, anions (phosphate, nitrate, sulfate, and arsenate), and cations (mercury, lead, and copper) in functionalized nanopores, and we compare their concentrations in nanopores with the corresponding bulk concentrations. Notably, chemical functional groups induced ion concentrations differently in nanopores. In pristine nanopores and methyl- and phenyl-functionalized nanopores, we discovered an unexpected concurrence of an enhanced anion concentration and a suppressed cation concentration. In addition, the nanopore pH is dependent on bulk solution compositions and can be lower by 2.5 units, even when the bulk solution is well-buffered. In contrast, for hydrophilic (amine, thiol, and carboxyl) nanopores, pH depended on the pKa of the functional groups, and the heavy metal concentrations depended on chemical interactions with the functional groups. Our findings provide a better understanding of water chemistry in nanopores and can help precisely control ions in nanopores to benefit the design of membrane-based desalination techniques, CO2 storage, and porous catalysts.