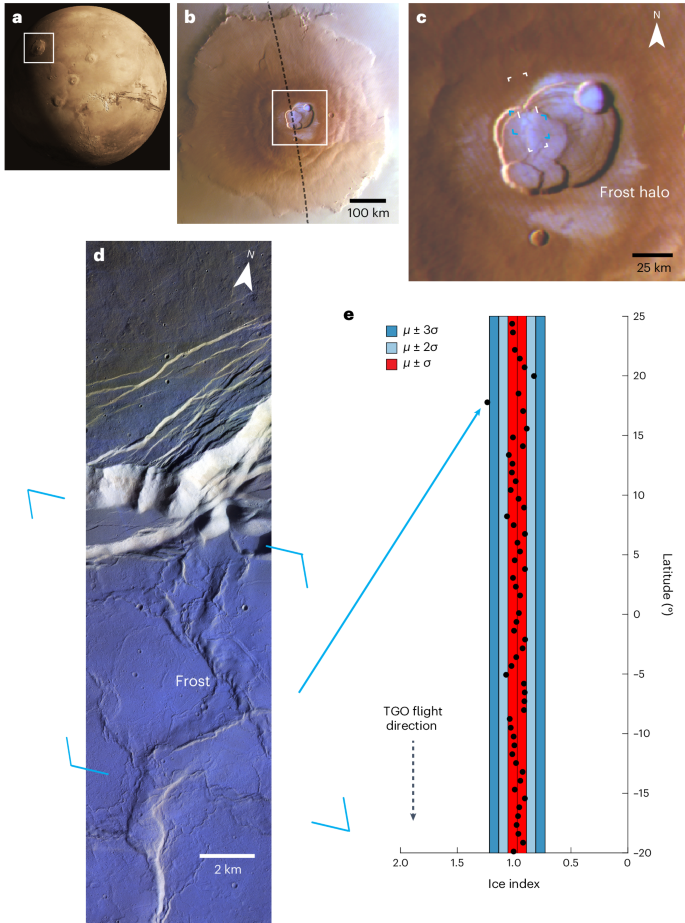
2024-06-10 米国国立再生可能エネルギー研究所(NREL)
<関連情報>
- https://www.nrel.gov/news/program/2024/advanced-spin-resonance-facility-fundamental-understanding-catalytic-mechanisms-structural-characterization-electrons.html
- https://pubs.aip.org/aip/jcp/article/159/23/235102/2930400/Low-temperature-trapping-of-N2-reduction-reaction
- https://pubs.acs.org/doi/10.1021/jacs.3c06832
ニトロゲナーゼMoFeタンパク質-CdS量子ドット複合体におけるN2還元反応中間体の低温捕捉に成功 Low-temperature trapping of N2 reduction reaction intermediates in nitrogenase MoFe protein–CdS quantum dot complexes
Lauren M. Pellows;Gregory E. Vansuch;Bryant Chica;Zhi-Yong Yang;Jesse L. Ruzicka;Mark A. Willis;Andrew Clinger;Katherine A. Brown;Lance C. Seefeldt;John W. Peters;Gordana Dukovic;David W. Mulder;Paul W. King
The Journal of Chemical Physics Published:December 20 2023
DOI:https://doi.org/10.1063/5.0170405

The biological reduction of N2 to ammonia requires the ATP-dependent, sequential delivery of electrons from the Fe protein to the MoFe protein of nitrogenase. It has been demonstrated that CdS nanocrystals can replace the Fe protein to deliver photoexcited electrons to the MoFe protein. Herein, light-activated electron delivery within the CdS:MoFe protein complex was achieved in the frozen state, revealing that all the electron paramagnetic resonance (EPR) active E-state intermediates in the catalytic cycle can be trapped and characterized by EPR spectroscopy. Prior to illumination, the CdS:MoFe protein complex EPR spectrum was composed of a S = 3/2 rhombic signal (g = 4.33, 3.63, and 2.01) consistent with the FeMo-cofactor in the resting state, E0. Illumination for sequential 1-h periods at 233 K under 1 atm of N2 led to a cumulative attenuation of E0 by 75%. This coincided with the appearance of S = 3/2 and S = 1/2 signals assigned to two-electron (E2) and four-electron (E4) reduced states of the FeMo-cofactor, together with additional S = 1/2 signals consistent with the formation of E6 and E8 states. Simulations of EPR spectra allowed quantification of the different E-state populations, along with mapping of these populations onto the Lowe–Thorneley kinetic scheme. The outcome of this work demonstrates that the photochemical delivery of electrons to the MoFe protein can be used to populate all of the EPR active E-state intermediates of the nitrogenase MoFe protein cycle.
光誘起CdS量子ドット-ニトロゲナーゼMoFeタンパク質複合体の低温アニールによりE4(2N2H)中間体の動力学的安定性が明らかになる Cryo-annealing of Photoreduced CdS Quantum Dot–Nitrogenase MoFe Protein Complexes Reveals the Kinetic Stability of the E4(2N2H) Intermediate
Gregory E. Vansuch, David W. Mulder, Bryant Chica, Jesse L. Ruzicka, Zhi-Yong Yang, Lauren M. Pellows, Mark A. Willis, Katherine A. Brown, Lance C. Seefeldt, John W. Peters, Gordana Dukovic, and Paul W. King
Journal of the American Chemical Society Published:September 20, 2023
DOI:https://doi.org/10.1021/jacs.3c06832
Abstract

A critical step in the mechanism of N2 reduction to 2NH3 catalyzed by the enzyme nitrogenase is the reaction of the four-electron/four-proton reduced intermediate state of the active-site FeMo-cofactor (E4(4H)). This state is a junction in the catalytic mechanism, either relaxing by the reaction of a metal bound Fe-hydride with a proton forming H2 or going forward with N2 binding coupled to the reductive elimination (re) of two Fe-hydrides as H2 to form the E4(2N2H) state. E4(2N2H) can relax to E4(4H) by the oxidative addition (oa) of H2 and release of N2 or can be further reduced in a series of catalytic steps to release 2NH3. If the H2 re/oa mechanism is correct, it requires that oa of H2 be associative with E4(2N2H). In this report, we have taken advantage of CdS quantum dots in complex with MoFe protein to achieve photodriven electron delivery in the frozen state, with cryo-annealing in the dark, to reveal details of the E-state species and to test the stability of E4(2N2H). Illumination of frozen CdS:MoFe protein complexes led to formation of a population of reduced intermediates. Electron paramagnetic resonance spectroscopy identified E-state signals including E2 and E4(2N2H), as well as signals suggesting the formation of E6 or E8. It is shown that in the frozen state when pN2 is much greater than pH2, the E4(2N2H) state is kinetically stable, with very limited forward or reverse reaction rates. These results establish that the oa of H2 to the E4(2N2H) state follows an associative reaction mechanism.