固体電池の短絡の仕組みを解明することで、電池の寿命を延ばせるかもしれない Understanding how short-circuits occur in solid-state batteries could extend their lifespan
2023-04-28 マックス・プランク研究所
研究者たちは、この有害な現象の成長過程を調べ、これを制御する方法を探っている。固体電池の結晶粒を短辺と長辺を持つレンガのように成長させ、電解質の粒界に沿って電子の流れを長くすることが可能であることが判明した。
電池の寿命を実際に延ばすことができるかどうかはまだ不明であるが、将来的に、固体電池が敏感な従来のリチウムイオン電池に取って代わる可能性はある。
<関連情報>
- https://www.mpg.de/19995577/solid-state-battery-lifespan?c=2249
- https://www.nature.com/articles/s41467-023-36792-7
オペランド顕微鏡法によるLi6.25Al0.25La3Zr2O12粒界におけるリチウムデンドライトの進展の解明 Understanding the evolution of lithium dendrites at Li6.25Al0.25La3Zr2O12 grain boundaries via operando microscopy techniques
Chao Zhu,Till Fuchs,Stefan A. L. Weber,Felix. H. Richter,Gunnar Glasser,Franjo Weber,Hans-Jürgen Butt,Jürgen Janek & Rüdiger Berger
Nature Communications Published:09 March 2023
DOI:https://doi.org/10.1038/s41467-023-36792-7
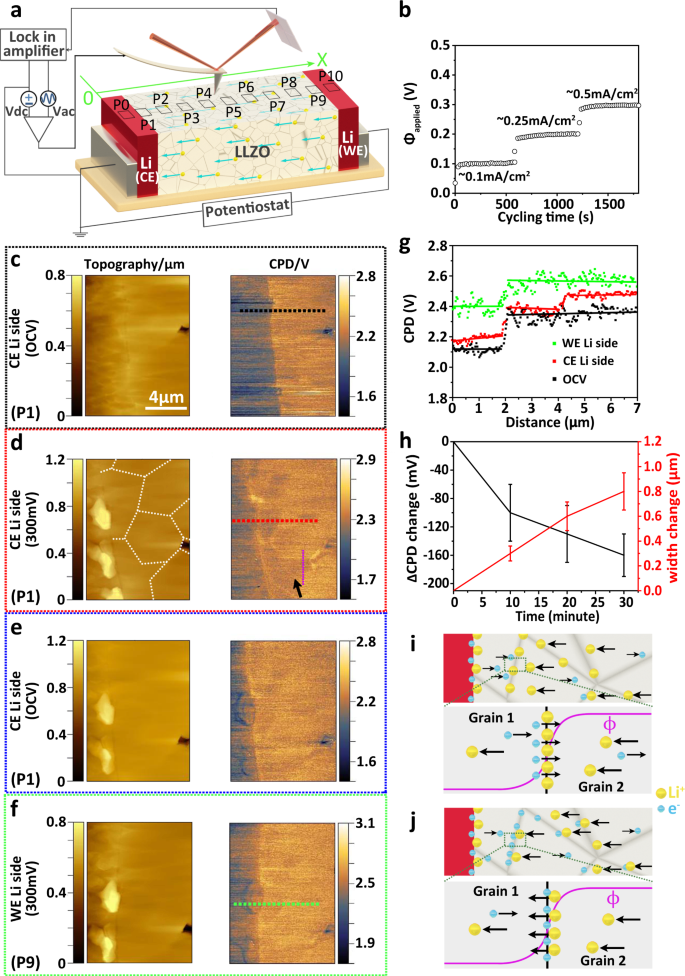
Abstract
The growth of lithium dendrites in inorganic solid electrolytes is an essential drawback that hinders the development of reliable all-solid-state lithium metal batteries. Generally, ex situ post mortem measurements of battery components show the presence of lithium dendrites at the grain boundaries of the solid electrolyte. However, the role of grain boundaries in the nucleation and dendritic growth of metallic lithium is not yet fully understood. Here, to shed light on these crucial aspects, we report the use of operando Kelvin probe force microscopy measurements to map locally time-dependent electric potential changes in the Li6.25Al0.25La3Zr2O12 garnet-type solid electrolyte. We find that the Galvani potential drops at grain boundaries near the lithium metal electrode during plating as a response to the preferential accumulation of electrons. Time-resolved electrostatic force microscopy measurements and quantitative analyses of lithium metal formed at the grain boundaries under electron beam irradiation support this finding. Based on these results, we propose a mechanistic model to explain the preferential growth of lithium dendrites at grain boundaries and their penetration in inorganic solid electrolytes.



