2026-01-07 中国科学院(CAS)
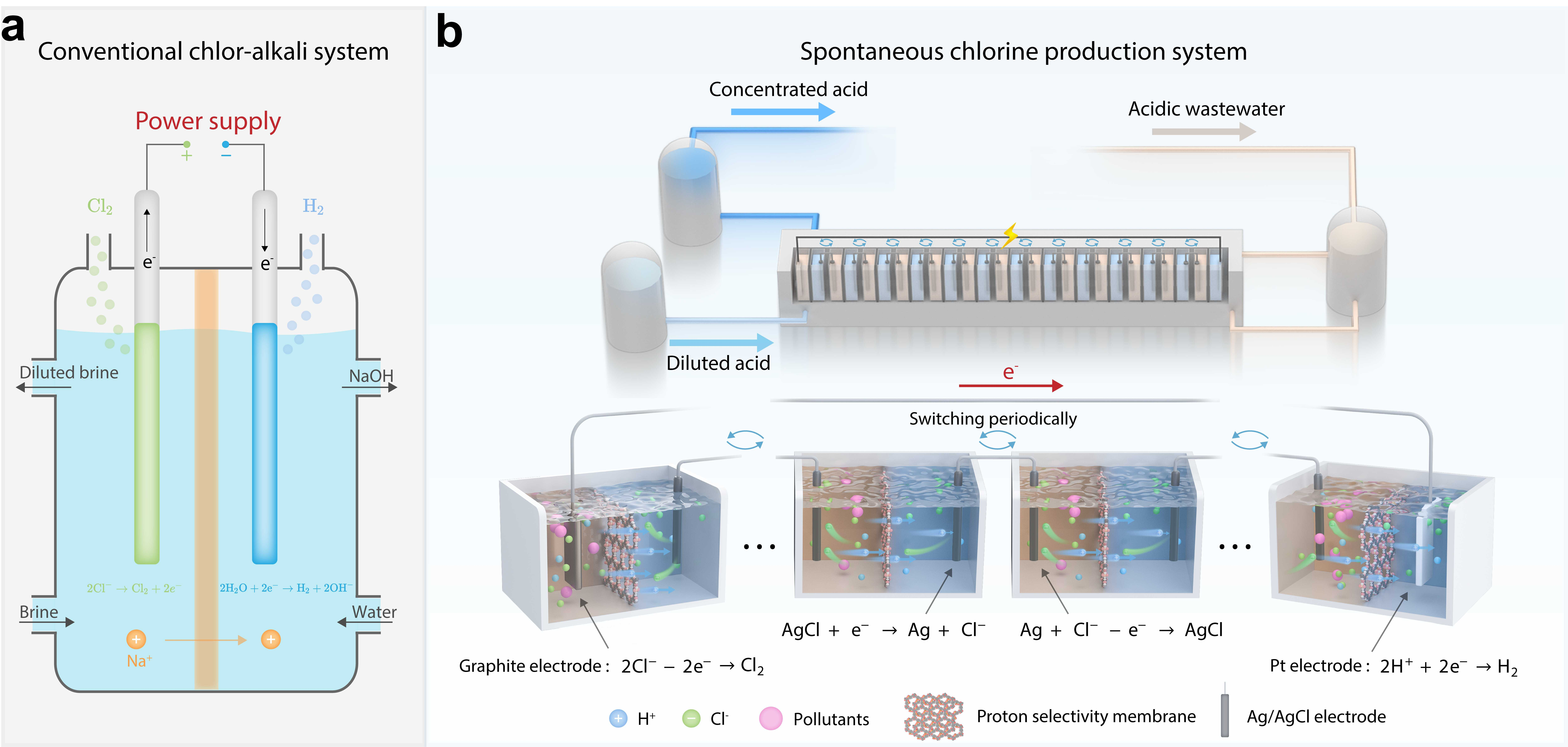
Schematic illustration of conventional chlor-alkali system and spontaneous chlorine production system. (Image by QIBEBT)
<関連情報>
- https://english.cas.cn/newsroom/research-news/202601/t20260107_1145328.shtml
- https://www.nature.com/articles/s41467-025-68181-7
塩化物含有塩水からの自然発生的な塩素生成 Spontaneous chlorine production from chloride-containing brines
Chenguang Zhu (朱晨光),Qi Li (李琪),Mingchang Li (李明昌),Shangfa Pan (潘尚发),Bo Zhou (周博),Lei Jiang (江雷) & Jun Gao (高军)
Nature Communications Published:07 January 2026
DOI:https://doi.org/10.1038/s41467-025-68181-7
Abstract
Chlorine, a crucial basic chemical, is primarily produced by the electrolysis of chloride-containing brines, a highly energy-intensive process with a substantial carbon footprint. Notably, concentrated chloride-containing brines, e.g., acidic wastewater, desalination wastewater, seawater, possess significant osmotic energy, which can be harnessed using membrane-based diffusion cells. Considering this, we here present a spontaneous chlorine production method by using the inherent energy and chloride ions present in these brines. The method is first demonstrated with simulated acidic wastewater because in industry, diffusion cells are already widely used to recycle waste acid. Sulfonated covalent-organic framework membranes are employed to facilitate the diffusion of protons and reject multi-valent cations, purifying acid and avoiding side reactions on the anodes. Consequently, our method simultaneously recovers acid, produces hydrogen and chlorine without consuming external energy. We also validate the general applicability of the method with simulated desalination wastewater. Since our method is compatible with the diffusion-based industrial processes, it holds significant promise for facile, scalable implementation. We also expect the method to be extended for the spontaneous production of other crucial chemicals such as ammonia from nitrate-containing brines.