2025-05-20 パシフィック・ノースウェスト国立研究所(PNNL)
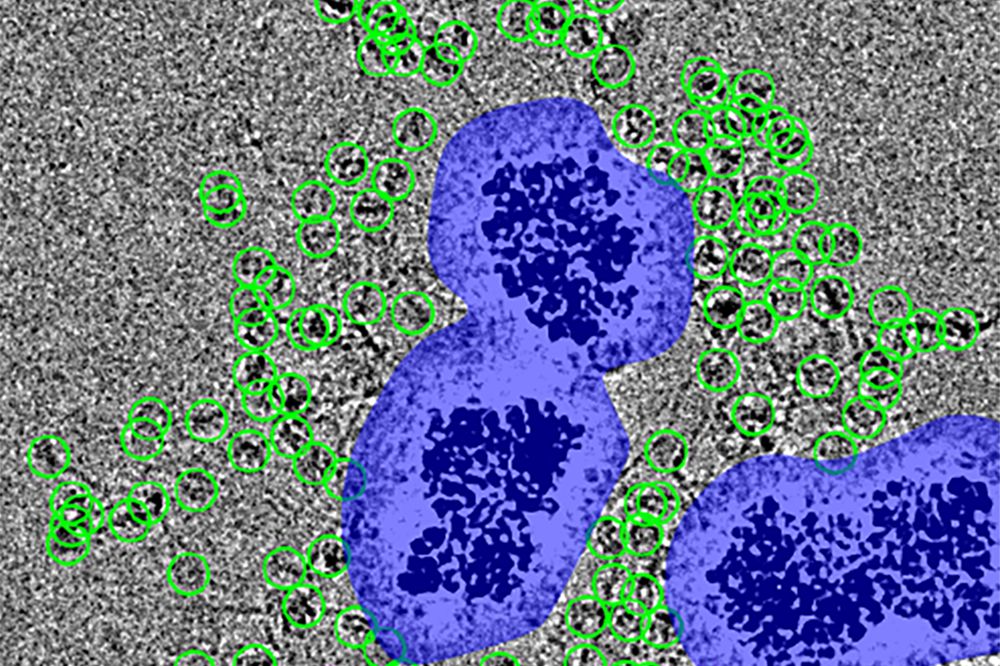
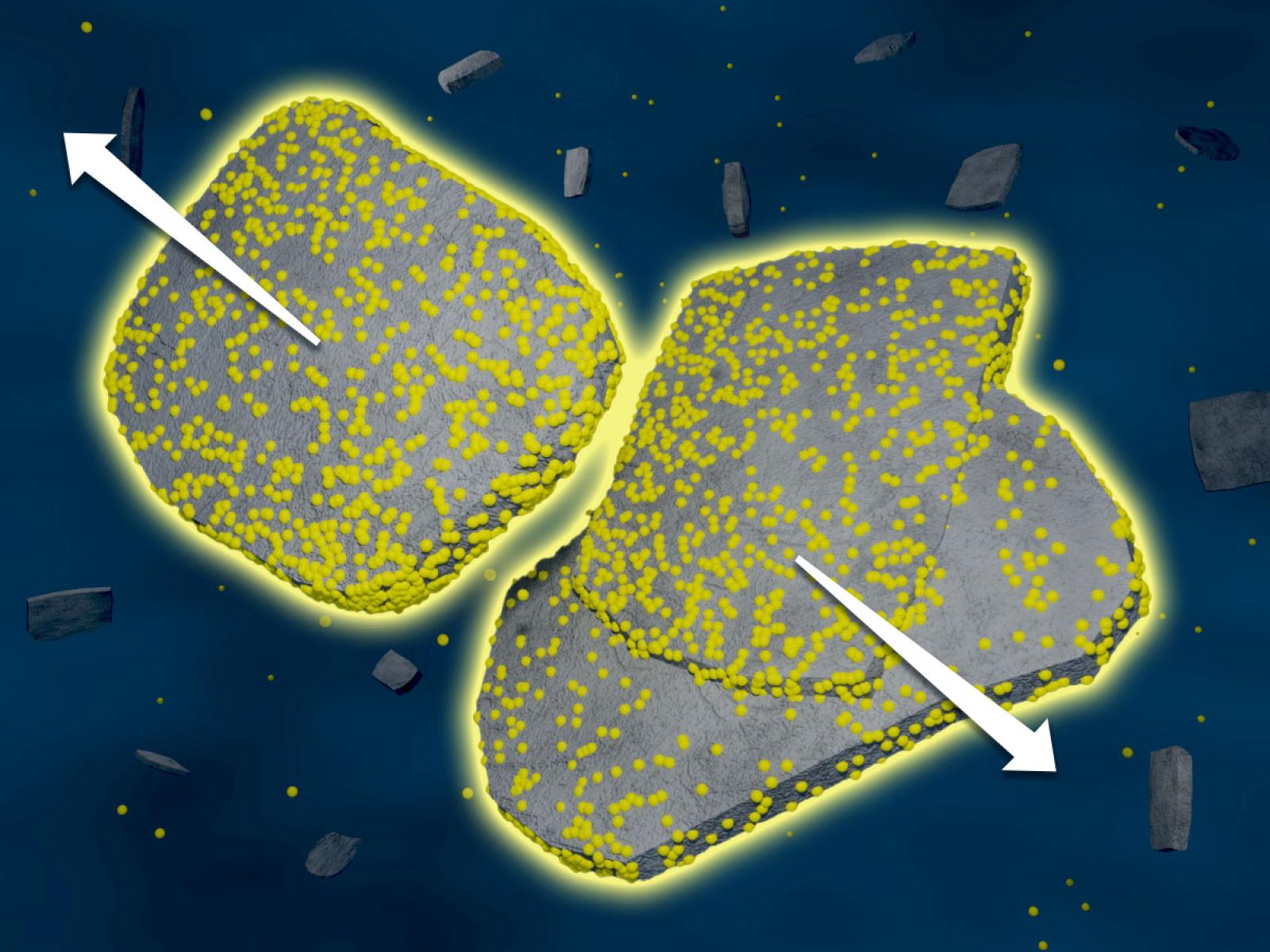 As the concentration of ions in a solution increases, nanoparticles are slower to aggregate, likely due to an increase in ions associated with particle surfaces. (Image by Nathan Johnson | Pacific Northwest National Laboratory)
As the concentration of ions in a solution increases, nanoparticles are slower to aggregate, likely due to an increase in ions associated with particle surfaces. (Image by Nathan Johnson | Pacific Northwest National Laboratory)
<関連情報>
- https://www.pnnl.gov/publications/ion-behavior-near-interfaces-affects-aggregation
- https://pubs.acs.org/doi/10.1021/acsnano.4c05563
イオン相関は界面での水和力を増加させることで粒子の凝集速度を低下させる Ion Correlations Decrease Particle Aggregation Rate by Increasing Hydration Forces at Interfaces
Pravalika Butreddy,Jaeyoung Heo,Nikhil Rampal,Tingting Liu,Lili Liu,William Smith,Xin Zhang,Micah P. Prange,Benjamin A. Legg,Gregory K. Schenter,James J. De Yoreo,Jaehun Chun,Andrew G. Stack,and Elias Nakouzi
ACS Nano Published: September 12, 2024
DOI:https://doi.org/10.1021/acsnano.4c05563
Abstract
The connection between solution structure, particle forces, and emergent phenomena at solid–liquid interfaces remains ambiguous. In this case study on boehmite aggregation, we established a connection between interfacial solution structure, emerging hydration forces between two approaching particles, and the resulting structure and kinetics of particle aggregation. In contrast to expectations from continuum-based theories, we observed a nonmonotonic dependence of the aggregation rate on the concentration of sodium chloride, nitrate, or nitrite, decreasing by 15-fold in 4 molal compared to 1 molal solutions. These results are accompanied by an increase in repulsive hydration forces and interfacial oscillatory features from 0.27–0.31 nm in 0.01 molal to 0.38–0.52 nm in 2 molal. Moreover, molecular dynamics (MD) simulations indicated that these changes correspond to enhanced ion correlations near the interface and produced loosely bound aggregates that retain electrolyte between the particles. We anticipate that these results will enable the prediction of particle aggregation, attachment, and assembly, with broad relevance to interfacial phenomena.