2024-08-29 カリフォルニア大学バークレー校(UCB)
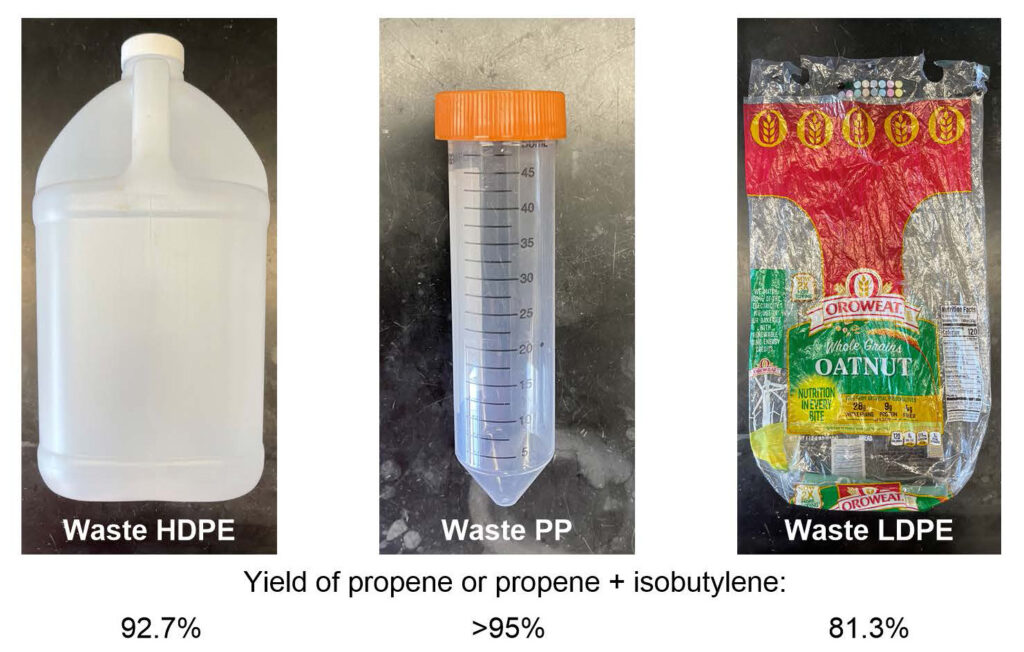 Examples of the types of plastics the new process can handle. Left to right, a jug made of high density polyethylene, a test tube of polypropylene and a low density polyethylene bread bag. The numbers below each image are the percentage yield of monomers that can be used to make new plastic polymers.
Examples of the types of plastics the new process can handle. Left to right, a jug made of high density polyethylene, a test tube of polypropylene and a low density polyethylene bread bag. The numbers below each image are the percentage yield of monomers that can be used to make new plastic polymers.
John Hartwig and RJ Conk, UC Berkeley
<関連情報>
- https://news.berkeley.edu/2024/08/29/new-process-vaporizes-plastic-bags-and-bottles-yielding-gases-to-make-new-recycled-plastics/
- https://www.science.org/doi/10.1126/science.adq7316
- https://www.science.org/doi/10.1126/science.add1088
- https://www.cell.com/chem/fulltext/S2451-9294(20)30603-3
ポリオレフィン廃棄物をエチレンと卑金属の不均一系触媒で軽オレフィンに変換 Polyolefin waste to light olefins with ethylene and base-metal heterogeneous catalysts
Richard J. Conk, Jules F. Stahler, Jake X Shi, Ji Yang, […], and John F. Hartwig
Science Published:29 Aug 2024
DOI:https://doi.org/10.1126/science.adq7316
Abstract
The selective conversion of polyethylene, polypropylene, and mixtures of the two polymers to form products with high volume demand is urgently needed because current methods suffer from low selectivity, produce large quantities of greenhouse gases, or rely on expensive, single-use catalysts. The isomerizing ethenolysis of unsaturated polyolefins could be an energetically and environmentally viable route to propylene and isobutylene, but noble-metal homogeneous catalysts and an unsaturated polyolefin are currently required, and the process has been limited to polyethylene. We show that the simple combination of tungsten oxide on silica and sodium on gamma-alumina transforms polyethylene, polypropylene, or a mixture of the two, including post-consumer forms of these materials, to propylene or a mixture of propylene and isobutylene in greater than 90% yield at 320°C without the need for dehydrogenation of the starting polyolefins.
廃ポリエチレンをエチレンで触媒分解してプロピレンを生成 Catalytic deconstruction of waste polyethylene with ethylene to form propylene
Richard J. Conk, Steven Hanna, Jake X. Shi, Ji Yang, […], and John F. Hartwig
Science Published:29 Sep 2022
DOI:https://doi.org/10.1126/science.add1088

Making propylene from polyethylene
Polyolefins are the most abundantly produced plastics but are unfortunately also among the hardest to break back down into their building blocks. Conk et al. developed a preliminary method to produce propylene from a sequence of reactions between waste polyethylene and fresh ethylene. Specifically, the process involves a small extent of desaturation of each polyethylene chain by a dehydrogenation catalyst, followed by steady breakdown of the chains into propylene through catalytic isomerization and metathesis reactions with ethylene. Subject to cost and process optimization, the method shows promise for generating a valuable feedstock chemical from waste plastic. —JSY
Abstract
The conversion of polyolefins to monomers would create a valuable carbon feedstock from the largest fraction of waste plastic. However, breakdown of the main chains in these polymers requires the cleavage of carbon–carbon bonds that tend to resist selective chemical transformations. Here, we report the production of propylene by partial dehydrogenation of polyethylene and tandem isomerizing ethenolysis of the desaturated chain. Dehydrogenation of high-density polyethylene with either an iridium-pincer complex or platinum/zinc supported on silica as catalysts yielded dehydrogenated material containing up to 3.2% internal olefins; the combination of a second-generation Hoveyda-Grubbs metathesis catalyst and [PdP(tBu)3(μ-Br)]2 as an isomerization catalyst selectively degraded this unsaturated polymer to propylene in yields exceeding 80%. These results show promise for the application of mild catalysis to deconstruct otherwise stable polyolefins.
ポリエチレンのC-H結合を選択的に触媒酸化することで、接着性を高めた機能性材料が得られる Selective, Catalytic Oxidations of C–H Bonds in Polyethylenes Produce Functional Materials with Enhanced Adhesion
Liye Chen, Katerina G. Malollari, Adam Uliana, Daniel Sanchez, Phillip B. Messersmith, John F. Hartwig
Chem Published:December 17, 2020
DOI:https://doi.org/10.1016/j.chempr.2020.11.020
Graphical abstract
The Bigger Picture
Hundreds of billions of pounds of polyolefins are manufactured per year. Despite this scale and widespread use of these materials, the applications of polyolefins have been limited by their lack of functional groups. The value of polyolefins arises, in part, from their resistance to chemical reactions. Thus, a grand challenge has been to modify polyolefins by selective functionalization of their C–H bonds.
We report the selective oxidation of polyethylenes to incorporate functional groups that imbue physical properties unseen in the unmodified materials. Although further work is needed to create catalysts that reach commercially suitable lifetimes with sustainable oxidants to fully realize this potential, our demonstration that these properties can be created by the direct modification of existing polyolefins enables one to reconsider the range of applications of these materials and modes for recycling polyolefin-based materials that could exploit the installed functionality.
Highlights
- Ruthenium-catalyzed oxidation of C–H bonds in polyethylenes with high turnovers
- Selective functionalization of commodity polyethylenes with varying architectures
- Processable and adhesive polyethylenes by post-polymerization modification
Summary
The synthesis of functional and processable polyethylenes from simple vinyl monomers with controlled molecular weights and architectures has been a grand challenge of polymer chemistry. Post-polymerization modification of the homopolymers of ethylene is attractive for this purpose but has been hampered by the lack of efficient methods for the selective functionalization of C–H bonds in polyolefins. We report the selective, catalytic oxidation of C–H bonds in commodity polyethylenes with varying molecular weights and architectures. Remarkably, the functionalized materials, even at low levels of functionalization, exhibit physical properties that are absent in unmodified polyolefins, such as strong adhesion and the ability to be painted with common waterborne latex paint. Such observations indicate that selectively modified polyethylenes as described here may help to transform existing commodity plastics into more valuable and potentially more sustainable materials.