2025-12-03 スウェーデン王立工科大学(KTH)
<関連情報>
- https://www.kth.se/en/om/nyheter/centrala-nyheter/study-shows-potential-for-more-affordable-and-efficient-hydrogen-gas-production-1.1444211
- https://www.nature.com/articles/s41557-025-01993-8
金属ヒドロキシル基は、異種O-O結合形成における分子内プロトン移動を媒介する Metal-hydroxyls mediate intramolecular proton transfer in heterogeneous O–O bond formation
Hao Yang,Fusheng Li,Shaoqi Zhan,Yawen Liu,Tianqi Liu,Linqin Wang,Wenlong Li,Mårten S. G. Ahlquist,Sumbal Farid,Rile Ge,Junhu Wang,Marc T. M. Koper & Licheng Sun
Nature Chemistry Published:14 November 2025
DOI:https://doi.org/10.1038/s41557-025-01993-8
Abstract
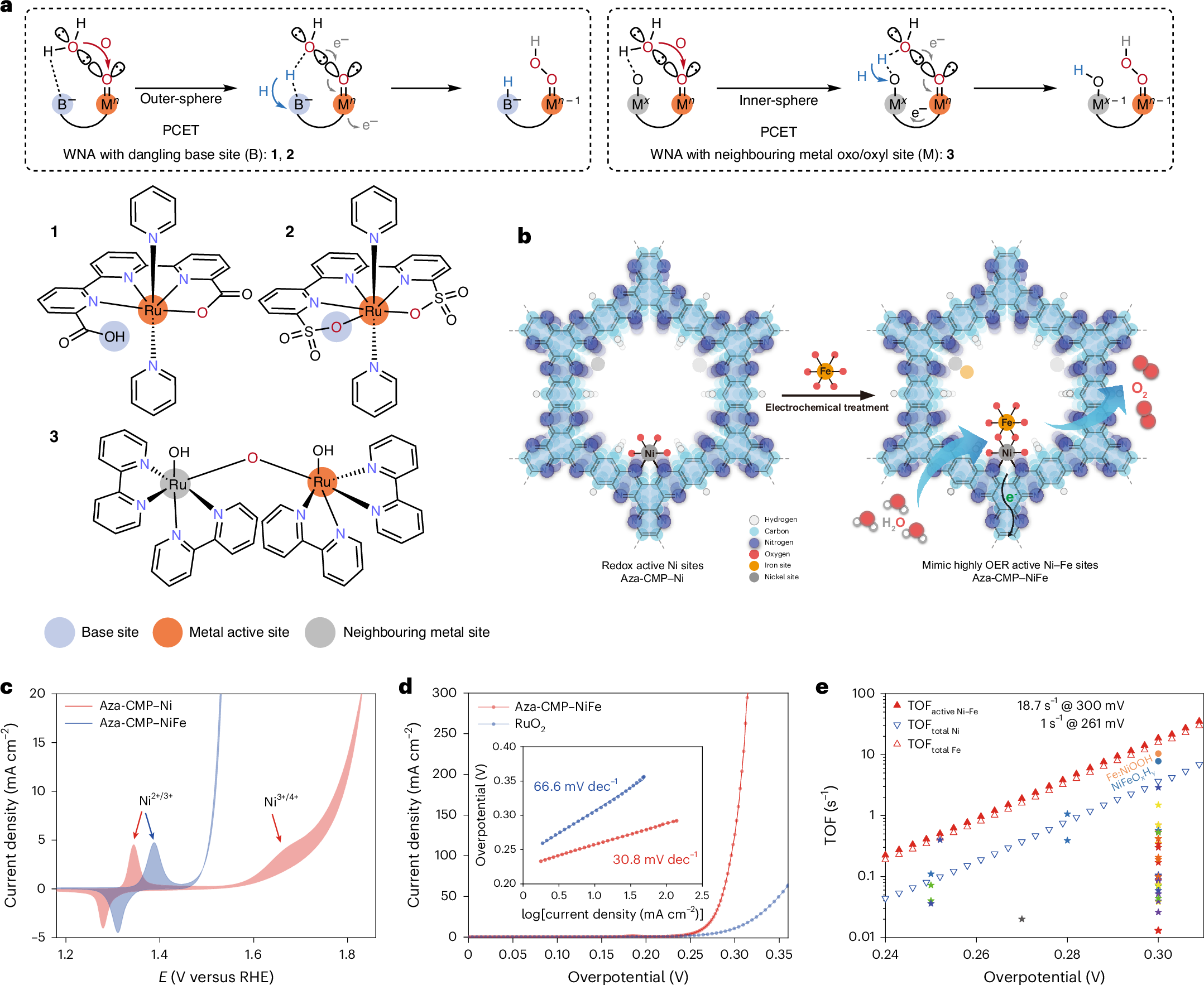
Metal (hydro)oxides are among the most effective heterogeneous water oxidation catalysts. Elucidating the interactions between oxygen-bridged metal sites at a molecular level is essential for developing high-performing electrocatalysts. Here we demonstrate that adjacent metal-hydroxyl groups function as intramolecular proton–electron transfer relays to enhance water oxidation kinetics. We achieved this using a well-defined molecular platform with an aza-fused π-conjugated microporous polymer that coordinates molecular Ni or Ni–Fe sites that emulate the structure of the most active edge sites in Ni–Fe materials for studying the heterogeneous water oxidation mechanism. We combine experimental and computational results to reveal the origin of pH-dependent reaction kinetics for O–O bond formation. We find both the anions in solution and the adjacent Ni3+–OH site act as proton transfer relays, facilitating O–O bond formation and leading to pH-dependent water oxidation kinetics. This study provides significant insights into the critical role of electrolyte pH in water oxidation electrocatalysis and enhancement of water oxidation activity in Ni–Fe systems.