2025-04-03 中国科学院(CAS)
<関連情報>
- https://english.cas.cn/newsroom/research_news/chem/202504/t20250402_909196.shtml
- https://www.nature.com/articles/s41586-025-08747-z
単原子触媒反応における金属-支持フロンティア軌道相互作用 Metal–support frontier orbital interactions in single-atom catalysis
Xianxian Shi,Zhilin Wen,Qingqing Gu,Long Jiao,Hai-Long Jiang,Haifeng Lv,Hengwei Wang,Jiani Ding,Mason P. Lyons,Alvin Chang,Zhenxing Feng,Si Chen,Yue Lin,Xiaoyan Xu,Pengfei Du,Wenlong Xu,Mei Sun,Yin Li,Bing Yang,Tao Zhang,Xiaojun Wu & Junling Lu
Nature Published:02 April 2025
DOI:https://doi.org/10.1038/s41586-025-08747-z
Abstract
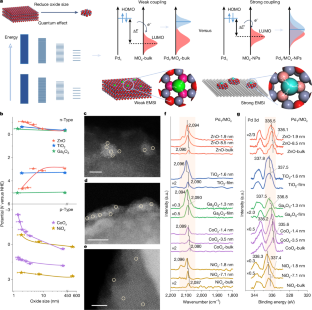
Single-atom catalysts (SACs) with maximized metal use and discrete energy levels hold promise for broad applications in heterogeneous catalysis, energy conversion, environmental science and biomedicine1,2,3,4,5,6,7. The activity and stability of SACs are governed by the pair of metal–adsorbate and metal–support interactions8,9,10. However, the understanding of these interactions with their catalytic performance in nature is challenging. Correlations of activity with the charge state of metal atoms have frequently reached controversial conclusions11,12,13,14,15. Here we report that the activity of palladium (Pd1) SACs exhibits a linear scaling relationship with the positions of the lowest unoccupied molecular orbital (LUMO) of oxide supports across 14 types of semiconductor. Elevation of the LUMO position by reducing the support particle size to a few nanometres boosts a record high activity along with excellent stability in the semi-hydrogenation of acetylene. We show that the elevated LUMO of support reduces its energy gap with the highest occupied molecular orbital (HOMO) of Pd1 atoms, which promotes Pd1–support orbital hybridizations for high stability and further amends the LUMO of anchored Pd1 atoms to enhance Pd1–adsorbate interactions for high activity. These findings are consistent with the frontier molecular orbital theory and provide a general descriptor for the rational selection of metal–support pairs with predictable activity.


