2024-11-14 カリフォルニア大学バークレー校(UCB)
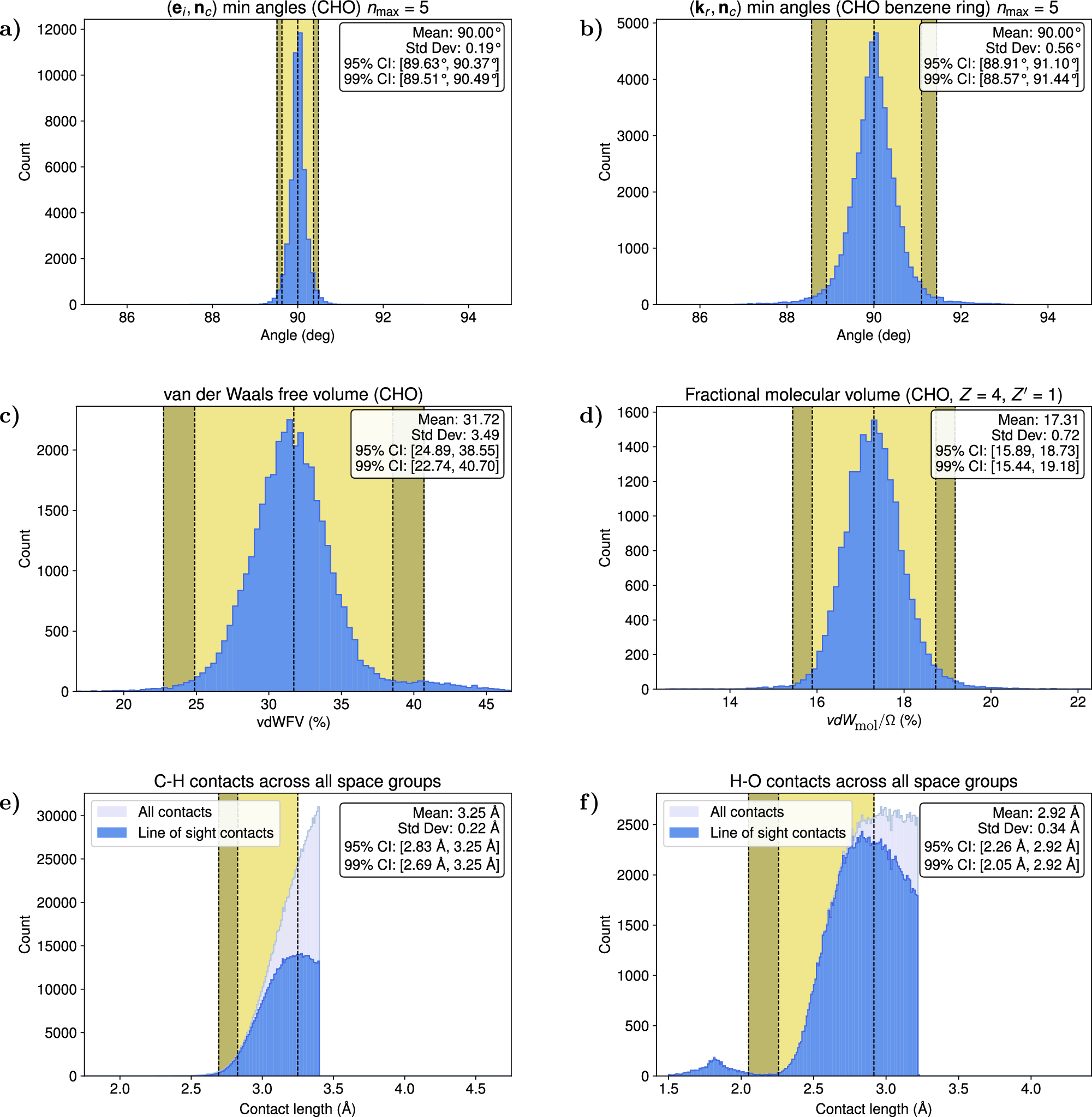
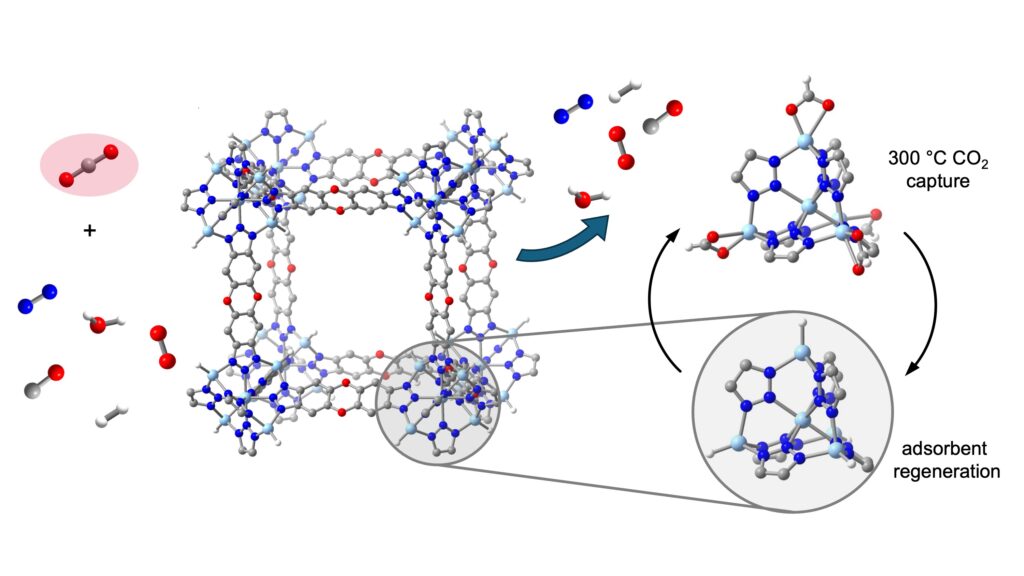 At center left is one of the crystalline building blocks of a thermally stable metal-organic framework (MOF), known as ZnH-MFU-4l, that is able to reversibly and selectively capture the greenhouse gas carbon dioxide from a mix of many industrially relevant gases. CO2 is highlighted at left, among nitrogen, oxygen, hydrogen, carbon monoxide and water molecules. The MOF can capture CO2 over many cycles at 300 C, which is a typical temperature of the exhaust streams from cement and steel plants. The zinc hydride groups in the MOF reversibly bind and release the carbon dioxide molecules (right). Light-blue, gray, blue, red, and white spheres represent Zn, C, N, O, and H atoms, respectively.
At center left is one of the crystalline building blocks of a thermally stable metal-organic framework (MOF), known as ZnH-MFU-4l, that is able to reversibly and selectively capture the greenhouse gas carbon dioxide from a mix of many industrially relevant gases. CO2 is highlighted at left, among nitrogen, oxygen, hydrogen, carbon monoxide and water molecules. The MOF can capture CO2 over many cycles at 300 C, which is a typical temperature of the exhaust streams from cement and steel plants. The zinc hydride groups in the MOF reversibly bind and release the carbon dioxide molecules (right). Light-blue, gray, blue, red, and white spheres represent Zn, C, N, O, and H atoms, respectively.
Rachel Rohde, Kurtis Carsch and Jeffrey Long, UC Berkeley
<関連情報>
- https://news.berkeley.edu/2024/11/14/breakthrough-in-capturing-hot-co2-from-industrial-exhaust/
- https://www.science.org/doi/10.1126/science.adk5697
末端水素化亜鉛サイトを持つ多孔質材料で高温二酸化炭素を回収 High-temperature carbon dioxide capture in a porous material with terminal zinc hydride sites
Rachel C. Rohde, Kurtis M. Carsch, Matthew N. Dods, Henry Z. H. Jiang, […], and Jeffrey R. Long
Science Published:14 Nov 2024
DOI:https://doi.org/10.1126/science.adk5697
Editor’s summary
Adsorption of CO2 by aqueous amines for carbon capture applications usually requires cooling of effluent gases to near-ambient temperatures. Rohde et al. found that zinc hydride sites in a metal-organic framework enabled the rapid capture of CO2 from high-temperature power generation and industrial processes (see the Perspective by Li and Zhao). Adsorption of CO2 to form zinc formate occurs reversibly through insertion into terminal zinc hydride sites. This process is slow at ambient temperatures but efficient at temperatures between 200°C and 400°C.—Phil Szuromi
Abstract
Carbon capture can mitigate point-source carbon dioxide (CO2) emissions, but hurdles remain that impede the widespread adoption of amine-based technologies. Capturing CO2 at temperatures closer to those of many industrial exhaust streams (>200°C) is of interest, although metal oxide absorbents that operate at these temperatures typically exhibit sluggish CO2 absorption kinetics and instability to cycling. Here, we report a porous metal–organic framework featuring terminal zinc hydride sites that reversibly bind CO2 at temperatures above 200°C—conditions that are unprecedented for intrinsically porous materials. Gas adsorption, structural, spectroscopic, and computational analyses elucidate the rapid, reversible nature of this transformation. Extended cycling and breakthrough analyses reveal that the material is capable of deep carbon capture at low CO2 concentrations and high temperatures relevant to postcombustion capture.