2024-07-08 テキサス大学オースチン校(UT Austin)
<関連情報>
- https://news.utexas.edu/2024/07/08/new-carbon-storage-technology-is-fastest-of-its-kind/
- https://pubs.acs.org/doi/10.1021/acssuschemeng.4c03809
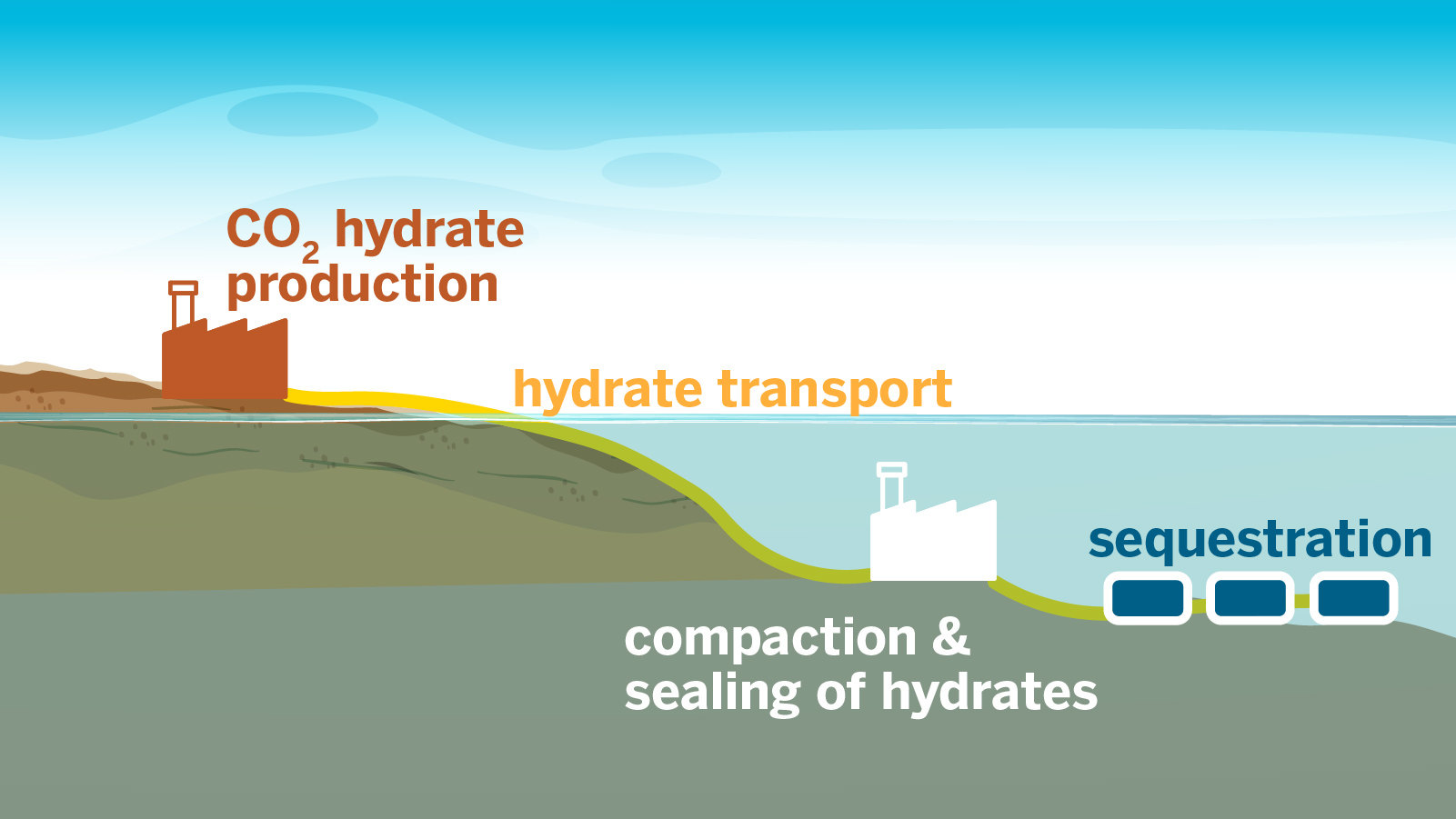
炭素貯留のための二酸化炭素ハイドレート泡の超高速形成 Ultrafast Formation of Carbon Dioxide Hydrate Foam for Carbon Sequestration
Awan Bhati, Mark Hamalian, Palash V. Acharya, and Vaibhav Bahadur
ACS Sustainable Chemistry & Engineering Published:July 8, 2024
DOI:https://doi.org/10.1021/acssuschemeng.4c03809
Abstract

We report ultrafast formation of carbon dioxide (CO2) hydrate foam without the use of any conventional chemical promoters or mechanical agitation. Our 6× enhancement in the CO2 sequestration rate (based on net gas consumption) results from the high flow rate sparging of CO2 gas in water in an open system (constant gas inflow/outflow) in the presence of magnesium. This approach continuously renews the gas–water–hydrate interface, thereby increasing the growth rate. The CO2 gas consumption rate (for hydrate foam formation) and foam composition (hydrate, CO2 dissolved in water, trapped CO2 gas) are experimentally quantified versus various parameters, including thermodynamic (pressure), CO2 flow-related parameters (flow rate, duration), water composition, and quantity of magnesium. The maximum measured CO2 sequestration rate (time-averaged) of 1276.5 g h–1 L–1 MPa–1 is 6 times higher than the fastest reported instantaneous rate. Importantly, we show rapid foam formation with saltwater, which will greatly improve the techno-economics. We develop an analytical framework to evaluate the composition of foam. We discover that the reactor pressure is a key determinant of the sequestration rate under high flow rate conditions, with magnesium playing a catalytic role. Overall, such foams enable new approaches to transport and sequester CO2 and benefit other applications that are hindered by notoriously sluggish hydrate formation.



